0102
Dynamic PRF and T1-based 3D Thermometry in the Liver using a Variable Flip Angle Stack-of-Radial Technique1Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Physics and Biology in Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 3Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
MR thermometry in the liver is challenged by mismatch between baseline and dynamic images caused by motion, leading to temperature errors. To address motion, previous methods had to compromise spatial coverage to increase temporal resolution. We propose a variable-flip-angle (VFA) 3D stack-of-radial technique for combined proton resonance frequency shift (PRF) and T1-based MR thermometry with volumetric coverage and high spatiotemporal resolution. Accurate VFA T1 calculation is achieved by synthesizing B1+ maps that match the liver position in dynamic images. A multi-baseline approach is used for accurate dynamic PRF measurements. Results from non-heating scans demonstrate reliable liver T1 and PRF measurements.
Introduction
Proton resonance frequency shift (PRF) is widely used to measure temperature during MR-guided thermal therapies1,2. However, when PRF is applied in the liver, breathing motion causes substantial error due to mismatch between dynamic and baseline images. To address this, a baseline image library can be collected before heating to cover the expected breathing motion range, and an appropriate baseline image can be selected to calculate temperature change at each time point. Previous studies3-5 have adopted this multi-baseline approach for fast liver PRF thermometry using echo-planar imaging sequences. However, these methods only provided limited through-plane coverage (1-5 slices) to achieve the temporal resolution needed to resolve breathing motion. We build on a previously-developed variable-flip-angle golden-angle-ordered (GA) 3D stack-of-radial sequence6 for dynamic PRF and T1-based MR thermometry in liver with 3D coverage and high spatiotemporal resolution . T1 can be used in conjunction with PRF as it is less susceptible to motion effects7. Moreover, T1 can provide temperature information in fatty tissues where PRF fails8.Methods
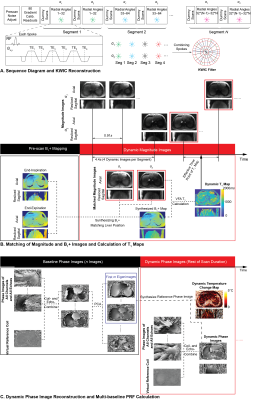
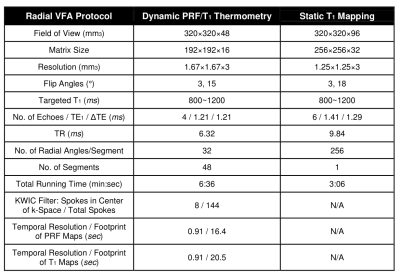
In an IRB-approved study, N=5 healthy subjects (3 males, 2 females) with age of 31±11 years and body mass index of 26.6±7.4kg/m2 underwent non-heating free-breathing abdominal scans at 3T (Prisma, Siemens) with body and spine arrays. Fig.1A shows the variable-flip-angle stack-of-radial PRF and T1 mapping sequence (“radial VFA”). After gradient calibration9 and dummy scans to establish steady state, segments of 32 GA radial angles were acquired with alternating flip angles. GA ordering continued across segments so that radial spokes can be grouped together during reconstruction, where a k-space weighted image contrast (KWIC) filter10 was applied.For dynamic radial VFA T1 mapping (Fig.1B), coil- and echo-combined magnitude images acquired with α1 were matched to those with α2 in neighboring segments with the highest similarity coefficient11. To calibrate flip angles, breath-held B1+ maps12 were acquired at end-expiration and end-inspiration. Dynamic B1+ maps were synthesized at each time point as a weighted sum of the two breath-held B1+ maps according to their similarity coefficient. A static T1 map was acquired using non-time-resolved radial VFA for comparison.
For PRF calculation (Fig.1C), dynamic phase images were reconstructed using virtual reference coils and echo-combined13 to an effective TE=10ms. The first n=60 sets of phase images (temporal footprint=71s) were grouped into a baseline library and principal component analysis (PCA) was performed14. The first m=6~10 principal vectors were retained such that their eigenvalues λ satisfied $$$\frac{\Sigma^{m}_{i=1}\lambda^2}{\Sigma^{n}_{i=1}\lambda^2}>0.99$$$ and could represent the expected range of motion. These m vectors were linearly combined to provide baseline images at each time point for PRF calculation14. Table 1 lists scanning parameters.
For comparison, dynamic T1 was also calculated by using only end-expiration B1+ maps and PRF was calculated using only a single baseline phase image acquired at end-expiration. To characterize the stability of radial VFA thermometry, the temporal coefficient of variation (COV) of T1 and temporal standard deviation (SD) of PRF temperature change were calculated on slices that were observed at all time points. One region of interest (ROI) of 2cm2 was drawn in each subject in one of these slices to track T1 and PRF fluctuations.
Results
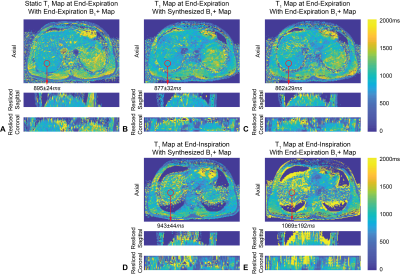
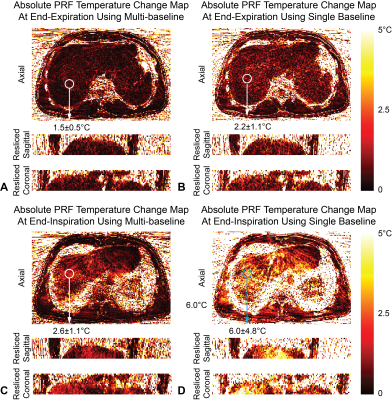
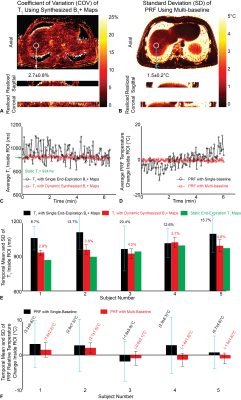
Fig.2A shows static T1 maps and Figs.2B and 2D show dynamic T1 maps calculated using synthesized dynamic B1+ maps. Figs.2C and 2E show that dynamic T1 maps using only end-expiration B1+ maps have errors due to misalignment. Figs.3A and 3C show absolute relative PRF temperature change maps calculated using the multi-baseline method, while Figs.3B and 3D show that PRF maps using a single end-expiration baseline have large errors especially towards the liver dome. Figs.4A and 4B show COV maps of T1 using synthesized B1+ maps and SD maps of PRF using multi-baseline, respectively, in one subject. The temporal fluctuations of mean T1 and PRF temperature inside the ROI in the same subject are plotted in Figs.4C and 4D. Figs.4E and 4F compare the temporal mean and SD of T1 and PRF inside five ROIs across all five subjects during entire scans. Synthesized B1+ maps reduced T1 COV substantially to an average of (3.9±0.6)% in ROIs across all subjects, while multi-baseline reduced PRF SD to (1.86±0.30)°C.Discussion
In non-heating free-breathing liver scans, our proposed radial VFA achieved 3D coverage (16 slices), in-plane resolution of 1.6x1.6mm2, and temporal resolution of <1s. The use of dynamic synthesized B1+ maps for VFA T1 calculation and multi-baseline PRF approach substantially improved the stability of both measurements compared to reference approaches. COV of T1 and SD of PRF temperature change were below 5% and 2.5°C, respectively, throughout the 6-minute scans even in the liver dome, which experienced prominent motion. Some blurring could be observed near liver boundaries due to the 15~20s temporal footprint of the KWIC filter. This could be reduced by incorporating parallel imaging acceleration15. Reference-less methods could also mitigate the need for baseline images and improve PRF accuracy4,16. The feasibility of using radial VFA to monitor temperature change should be evaluated in future thermal ablation experiments.Conclusion
In vivo non-heating evaluations of our proposed variable-flip-angle GA stack-of-radial PRF-T1 thermometry technique in the liver demonstrated good stability with dynamic 3D coverage and high spatiotemporal resolution. This technique has the potential to improve monitoring/control of thermal therapies in moving organs during free-breathing.Acknowledgements
This study was supported in part by Siemens Healthineers and UCLA Radiological Sciences. The authors thank the study coordinators at UCLA.References
1. Merckel LG, Knuttel FM, Deckers R et al. First clinical experience with a dedicated MRI-guided high-intensity focused ultrasound system for breast cancer ablation. Eur Radiol. 2016;26(11):4037-4046
2. Rouviere O, Gelet A, Crouzet S et al. Prostate focused ultrasound focal therapy—imaging for the future. Nat Rev Clin Oncol. 2012;9:721-727
3. de Senneville BD, Roujol S, Moonen C et al. Motion correction in MR thermometry of abdominal organs: a comparison of the referenceless vs. the multibaseline approach. Magn Reson Med. 2010;64(5):1373-1381
4. Grissom WA, Rieke V, Holbrook AB et al. Hybrid referenceless and multibaseline subtraction MR thermometry for monitoring thermal therapies in moving organs. Med Phys. 2010;37(9):5014-5026
5. Bour P, Ozenne V, Marquet F et al. Real-time 3D ultrasound-based motion tracking for the treatment of mobile organs with MR-guided high-intensity focused ultrasound. Int J Hyperthermia. 2018;34(8):1225-1235
6. Zhang L, Armstrong T, Li X et al. A variable flip angle golden-angle-ordered 3D stack-of-radial MRI technique for simultaneous proton resonant frequency shift and T1-based thermometry. Magn Reson Med. 2019;82(6):2062-2076
7. Todd N, Diakete M, Payne A et al. Hybrid Proton Resonance Frequency/T1 Technique for Simultaneous Temperature Monitoring in Adipose and Aqueous Tissues. Magn Reson Med. 2013;69(1):62-70
8. Hynynen K, McDannold N, Mulkern RV et al. Temperature monitoring in fat with MRI. Magn Reson Med 2000;43(6):901–904
9. Armstrong T, Dregely I, Stemmer A et al. Free-breathing liver fat quantification using a multiecho 3D stack-of-radial technique. Magn Reson Med. 2018;79(1):370-382
10. Song H, Dougherty L. k-Space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med. 2000;44(6):825-832
11. Roujol S, Ries M, Quesson B et al. Real-time MR-thermometry and dosimetry for interventional guidanceon abdominal organs. Magn Reson Med. 2010;63(4):1080–1087
12. Chung S, Kim D, Breton E et al. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med. 201;64(2):439-446
13. Svedin B, Payne A, Bolster B et al. Multiecho pseudo-golden angle stack of stars thermometry with high spatial and temporal resolution using k-space weighted image contrast. Magn Reson Med. 2018;79(3):1407-1419
14. Tan J, Mougenot C, Pichardo S et al. Motion compensation using principal component analysis and projection onto dipole fields for abdominal magnetic resonance thermometry. Magn Reson Med. 2019;81(1): 195-207
15. Uecker M, Lustig M. Estimating absolute-phase maps using ESPIRiT and virtual conjugate coils. Magn Reson Med. 2017;77(3):1201-1207
16. Grissom WA, Lustig M, Holbrook AB et al. Reweighted ℓ1 referenceless PRF shift thermometry. Magn Reson Med. 2010;64(4):1068-1077
Figures