0098
Automatic detection and reacquisition of motion degraded images in fetal HASTE imaging at 3T1Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children's Hospital, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3(co-first author) Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 5Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 6Department of Electrical Engineering, Princeton University, Princeton, NJ, United States, 7Siemens Medical Solutions USA, Inc, Charlestown, MA, United States, 8Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 9Computer Science and Artificial Intelligence Laboratory (CSAIL), Massachusetts Institute of Technology, Cambridge, MA, United States, 10Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Fetal brain MRI suffers from unpredictable and unconstrained fetal motion that not only causes severe image artifacts even with single-shot FSE readouts, but also results in slice-to-slice variations of the imaging plane and long scanning sessions, as the MR technologist “chases” the fetal head in an attempt to acquire artifact-free orthogonal images. In this work, we have implemented a closed-loop pipeline that automatically detects and reacquires HASTE images that were degraded by fetal motion, without any interaction from the MRI technologist. The presented methods demonstrate the basic infrastructure needed for successful prospective automated fetal brain motion correction.
Introduction/Purpose
Fetal brain MRI suffers from unpredictable fetal motion, limiting the types of MR acquisition schemes practical for use in clinical settings to fast single-shot encoding techniques, such as HASTE. Nevertheless, even when using HASTE, fetal MRI sessions can be significantly lengthened, as fetal motion during the HASTE acquisition not only degrades image quality, but also introduces slice-to-slice variations of the imaging plane, resulting in double-oblique slices which are hard to interpret clinically. Therefore, in a typical fetal MRI session, the MRI technologist “chases” the fetal head, in an attempt to obtain images in the standard three orthogonal planes, resulting in repetition of the entire HASTE acquisition if sufficient slices in the stack are motion degraded or became double oblique. Thus, the current fetal imaging workflow depends on rapid assessment of the image stack quality and determination if it should be repeated. In this work, we designed and implemented a prototype closed-loop acquisition/reconstruction pipeline that automatically, without human interaction, detects and re-acquires only the slices that have been degraded by fetal motion, and not the whole stack.Methods
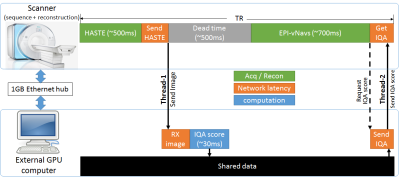
We have previously shown the feasibility of using a convolutional neural network (CNN) engine that performs an automatic image quality assessment (IQA) of fetal brain HASTE images, and outputs a quality score1. In this work we developed and implemented a pipeline that runs the IQA CNN engine on a GPU (NVIDIA 1050Ti) equipped computer, connected to the scanner’s internal network via 1GB Ethernet hub, for efficient communication/feedback between this computer and the scanner's computer running our custom MRI sequence and reconstruction. This setup is similar to those used for real-time neurofeedback in fMRI experiments2–4 and prospective motion correction in neuroanatomical MRI5,6. The proposed methods involved modifying the HASTE sequence acquisition and reconstruction, as well as generating Python scripts that in real time receive the HASTE images, run the CNN IQA engine, and send the IQA score of each slice back to the sequence. Figure 1 shows a schematic of the overall acquisition/reconstruction engine, described below.HASTE acquisition modification: We used an enhanced HASTE sequence, called vNav-HASTE, which embeds low resolution EPI volumetric navigators (EPI-vNavs)7 within the TR to obtain a low resolution volume of the fetal head (5mm3 voxels, 3D EPI readout, TA=0.7s) before every acquired HASTE slice8. For this project, we further modified this sequence to enable: 1. socket connection to the GPU computer for seamless transfer of the IQA scores upon sequence request during run-time; 2. reacquisition loop that starts seamlessly (without any human interaction) right after the prescribed HASTE stack is acquired, and reacquires a user defined number of slices, NREACQ, with the NREACQ worst IQA scores.
HASTE reconstruction modification: The online reconstruction was modified such that HASTE images are sent to the GPU computer via a socket connection as soon as they are reconstructed. Additionally, besides the original HASTE stack, the reconstruction engine outputs the NREACQ reacquired images in a separate image series.
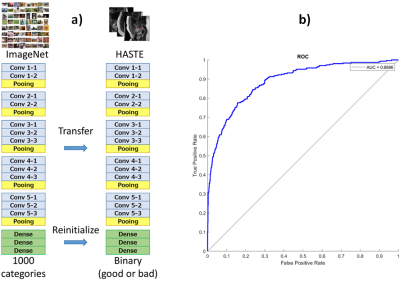
IQA scores calculation: We trained a VGG-16 network (Figure 2a) to classify HASTE images as diagnostic or non-diagnostic. The network is first pre-trained on Imagenet dataset and then fine-tuned on a fetal HASTE dataset (4432/1557 diagnostic/nondiagnostic images). To address the problem of class imbalance in MRI dataset, we adopted weighted binary cross entropy as loss function during fine-tuning. The network was evaluated on a separate test set with 1329 slices, see ROC curve in Figure 2b. With an image with size of 256x256, each IQA score is computed in 30 ms on the GPU computer used.
Phantom development and in vivo fetal scans were performed on a 3T Skyra scanner (Siemens Healthcare, Erlangen, Germany) using spine and body flex receive arrays. The vNav-HASTE-reacq sequence (TE/TR=119ms/1.8s, FOV=33x33cm2, 1.3x1.3x3mm3 voxels, PF=5/8, RGRAPPA=2) was run on two pregnant mothers who signed informed consent forms approved by BCH’s IRB.
Results
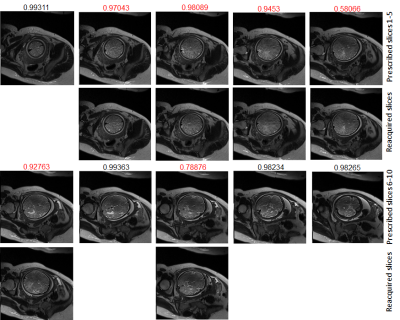
Figure 3 shows images from vNav-HASTE-reacq scan with N=10 slices and NREACQ=6 slices to reacquire, showing that our sequence correctly reacquired the slices at locations where the 6 slices with the lowest IQA scores (shown in red) were originally acquired. Figure 4 shows 4 images from 3 separate scans, where the originally acquired slices were motion degraded, and the re-acquired ones were not. The IQA scores shown above the images range from 0 to 1 (0=poor, 1=excellent quality).Discussion/Conclusion
We have demonstrated a closed-loop acquisition/reconstruction pipeline that automatically detects and reacquires motion degraded fetal brain HASTE images. The proposed infrastructure has all the necessary infrastructural capabilities for robust real-time, prospective fetal head motion correction. Specifically, our future work includes developing a computational engine capable of efficiently and reliably determining the fetal head pose from the low resolution EPI-vNavs obtained before each HASTE slice. The communication framework described here can easily be adapted to include this information for appropriate reorientation of the FOV of the next HASTE slice. Moreover, we intend to further improve the accuracy performance of the IQA CNN engine. This work is part of our greater effort and ultimate goal of developing an intelligent system that “chases” the fetal head in real-time to obtain high quality, high resolution, diagnostic HASTE images of the entire brain in the least amount of time.Acknowledgements
NIH R01 EB017337, U01 HD087211, R01HD100009, R00HD074649, R01HD099846, R01HD093578, R01HD085813, The NVIDIA Corporation.References
1 Lala S, Singh N, Gagoski B, Turk E, Grant PE, Golland P, Adalsteinsson E. A Deep Learning Approach for Image Quality Assessment of Fetal Brain MRI. 27th Annual Proceedings of the International Society for Magnetic Resonance in Medicine 2017.
2 Hinds O, Ghosh S, Thompson TW, Yoo JJ, Whitfield-Gabrieli S, Triantafyllou C, et al. Computing moment-to-moment BOLD activation for real-time neurofeedback. Neuroimage 2011;54:361–8.
3 Lee J, Wighton P, Cauley SF, Setsompop K, van der Kouwe A, Loggia ML, et al. Application of Simultaneous Multi-Slice (SMS) Imaging to Real-time fMRI for Improved Neurofeedback Signal Fidelity. Real-Time Functional Imaging and Neurofeedback (rtFIN) Conference 2015.
4 Wighton P, Karahanoglu FI, Tisdall, van der Kouwe A. Slice-by-Slice prospective motion correction in EPI using a markerless motion tracking system. International Society for Magnetic Resonance in Medicine’s (ISMRM) Workshop on Motion Correction in MRI 2017.
5 Gilman J, Wighton P, Curran MT, Lee S, Thompson T, De Los Angeles CS, et al. Modulation of Visual Attention of Blended Faces and Scenes in the FFA and PPA. Real-Time Functional Imaging and Neurofeedback (rtFIN) Conference 2015.
6 Frost R, Wighton P, Karahanoğlu FI, Robertson RL, Grant PE, Fischl B, et al. Markerless high-frequency prospective motion correction for neuroanatomical MRI. Magn Reson Med 2019;82:126–44.
7 Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med 2012;68:389–99.
8 Gagoski B, McDaniel P, van der Kouwe A, Bhat H, Wald L, Adalsteinsson E, et al. HASTE Imaging with EPI Volumetric Navigators for Real-Time Fetal Head Motion detection. 24th Annual Proceedings of the International Society for Magnetic Resonance in Medicine 2016.
Figures