0095
Fetal and neonatal whole brain T2* mapping at 3T
Serge Vasylechko1, Emer Hughes2, Joanna Allsop2, Matthew Fox2, Daniel Rueckert1, and Jo Hajnal2
1Biomedical Image Analysis Group, Department of Computing, Imperial College London, London, United Kingdom, 2Centre for the Developing Brain, School of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom
1Biomedical Image Analysis Group, Department of Computing, Imperial College London, London, United Kingdom, 2Centre for the Developing Brain, School of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom
Synopsis
Quantitative T2* mapping in the developing brain is challenging due to inherent motion of fetal and neonatal subjects. This study uses a motion robust framework for acquisition, reconstruction and segmentation of whole brain T2* maps. This is achieved by single-shot multi-echo GRE EPI acquisition, multi-level slice-to-volume registration and gestational-age specific brain atlas segmentation. T2* values are reported for fetal and neonatal subjects at 3T. Findings indicate large variability in T2* within each subject group, non-linear change in T2* between fetal and preterm neonatal period, and significantly higher mean T2* constants than previously reported in adult subjects.
Introduction
Changes to T2* relaxation time constants correlate with changes in myelo- and cyto- architecture 1 and could help with evaluation of pathology and normal brain development. Knowledge of T2* constants is an important component for optimization of sensitivity to BOLD in fMRI 2. In this study normative values for T2* constants were measured at 3T in fetal and neonatal subjects across the whole brain. Acquisition of quantitative T2* maps is challenging due to maternal and fetal motion in fetal MRI, and sporadic motion in neonatal MRI during natural sleep. We extend a previously described method that introduced motion robust acquisition for reconstruction of T2* maps in the fetal brain 3 with a post-processing framework that achieves a fully automatic segmentation of whole brain T2* maps. Consequently, we are able to report measurements of T2* constants in 14 regions of interest (ROI) that cover the entire brain, rather than measurements on manually selected seed voxels in limited number of ROIs that were reported in previous studies 3,4,5,6,7.Methods
Single-shot multi-echo gradient echo EPI was used to effectively freeze intra-slice and inter-echo motion during acquisition as presented in Vasylechko et al 3. Successive images of each slice at different echo times could therefore be combined to measure T2* maps on a slice by slice basis. Individual T2* weighted slices were mapped to a 3D reconstructed T2 weighted brain volume via an iterative multi-resolution registration scheme. Automatic segmentation of the brain into 14 ROIs was achieved with gestational age (GA) specific atlases in the method described by Makropoulos et al 8. T2* estimates were corrected for through-slice susceptibility induced background gradients in the neonatal images 9.Acquisition protocols were:
Fetal: TE 36ms, ΔTE 50ms, 7 echoes, 3mm3 isotropic resolution, FOV 320x320, 43 slices, SENSE 2, TR 10s.
Neonatal: TE 50ms, ΔTE 80ms, 7 echoes, 2.5mm3 isotropic resolution, FOV 270x180, 30 slices, SENSE 2.5, TR 13s.
Scans were preceded by a clinical examination, including T2 weighted single shot turbo spin echo acquisition in 3 orthogonal planes, which allowed for 3D reconstruction of high resolution brain anatomy 10. The subjects consisted of 10 fetal, 8 preterm and 8 preterm scanned at term, with no evidence of focal abnormalities. In each case written informed consent was obtained. The median (range) GA were 32.5 (30-34), 34 (30-36) and 41.5 (41-43) weeks respectively.
Results
Mean T2* in the fetal group were higher than in preterm neonates for all ROIs. Mean T2* in the preterm group were in turn higher than in preterm at term group for all ROIs. These trends are consistent with the previous studies at 1.5T for the 4 ROIs that were previously reported 3,4,6,7. T2* measurements of the cerebral cortex, both GM and WM, were higher in all subdivided ROIs for all groups, than the structures in the diencephalon and the brainstem.Significant statistical difference was found between fetal and preterm subjects for 7 ROIs. ANOVA analysis between preterm and preterm at term subjects had shown no significant difference in any ROIs, which is consistent with the limited ROI measurements in Rivkin et al 6, but is different to Lee et al 7 due to GA difference. Assessment of coefficient of variation (CoV) for fetal, preterm and preterm at term groups was lower or equal to CoV in previous studies at 1.5T 3,4,6,7.
Non-zero correlation between T2* constants and gestational age was found in all ROIs with p-values not exceeding 0.01 in any region. However, the R2 had not exceeded 0.25 in any regions except for basal ganglia (R2 = 0.51) and thalamus (R2 = 0.46). This suggests that, while the correlation between T2* decay constants and gestational age are significant, the measure of variance in T2* decay estimates is not well modelled by linear regression by gestational age alone.
T2* estimates in our study were significantly higher than those of Goksan et al 5 at 3T for comparable ROIs. This difference may be attributed to the different clinical groups - preterm at term subjects in our study and term infants in Goksan et al 5.
Discussion & Conclusions
Our findings indicate that T2* constants differ significantly between fetal and preterm born neonatal subjects in the cortical areas and cerebellum. The balance between intrinsic tissue differences and differences between the in utero and ex utero environment in contributing to these differencs is uncertain. Relative changes in T2* decay constants between fetal and postnatal transition phases are strongest in the occipital lobe, temporal lobe and cerebellum, which are responsible for sensory and motor functions of the brain, and are in line with asynchronous development patterns reported in post-mortem studies 11.Notably, variation between T2* constants in the cortical regions are much larger in fetal and preterm subjects than in adults. Careful considerations should be made for TE values in fMRI protocols with respect to a specific ROI being measured to ensure robust BOLD sensitivity.
Acknowledgements
This work was supported by the MRC through a strategic grant and EPSRC grant.References
- Cohen-Adad, J. (2014). “What can we learn from T2* maps of the cortex?”. Neuroimage 93, pp. 189–200.
- Chavhan, G. B., Babyn, P. S., Thomas, B., Shroff, M. M., and Haacke, E. M. (2009). “Principles, techniques, and applications of T2*-based MR imaging and its special applications”. Radiographics 29.5, pp. 1433–1449.
- Vasylechko, S., Malamateniou, C., Nunes, R. G., Fox, M., Allsop, J., Rutherford, M., Rueckert, D., and Hajnal, J. V. (2015). “T2* relaxometry of fetal brain at 1.5 Tesla using a motion tolerant method”. Magnetic Resonance in Medicine 73.5, pp. 1795–1802.
- Blazejewska, A. I., Seshamani, S., McKown, S. K., Caucutt, J. S., Dighe, M., Gatenby, C., and Studholme, C. (2017). “3D in utero quantification of T2* relaxation times in human fetal brain tissues for age optimized structural and functional MRI”. Magnetic Resonance in Medicine 78.3, pp. 909–916.
- Goksan, S., Hartley, C., Hurley, S.A., Winkler, A.M., Duff, E.P., Jenkinson, M., Rogers, R., Clare, S. and Slater, R. (2017). “Optimal echo time for functional MRI of the infant brain identified in response to noxious stimulation”. Magnetic Resonance in Medicine 78(2), pp.625-631.
- Rivkin, M. et al. (2004). “Prolonged T*2 values in newborn versus adult brain, Implications for fMRI studies of newborns”. Magnetic Resonance in Medicine 51.6, pp. 1287–91.
- Lee, W., Donner, E., Nossin-Manor, R., Whyte, H., Sled, J., and Taylor, M. (2012). “Visual functional Magnetic Resonance Imaging of preterm infants”. Developmental Medicine and Child Neurology 54.8, pp. 724–9.
- Makropoulos, A., Gousias, I. S., Ledig, C., Aljabar, P., Serag, A., Hajnal, J. V., Edwards, A. D., Counsell, S. J., and Rueckert, D. (2014). “Automatic whole brain MRI segmentation of the developing neonatal brain”. IEEE Transactions On Medical Imaging 33.9, pp. 1818–1831.
- Dahnke, H. and Schaeffter, T. (2005). “Limits of detection of SPIO at 3.0 T using T2* relaxometry”. Magnetic Resonance in Medicine 53.5, pp. 1202–1206.
- Kainz, B. et al. (2015). “Fast volume reconstruction from
motion corrupted stacks of 2D slices”. IEEE Transactions On Medical Imaging 34.9,
pp. 1901–1913.
- Dubois, J., Dehaene-Lambertz, G., Kulikova, S., Poupon, C.,
Hüppi, P. S., and Hertz-Pannier, L. (2014). “The early development of brain
white matter: a review of imaging studies in fetuses, newborns and infants”.
Neuroscience 276, pp. 48–71.
Figures
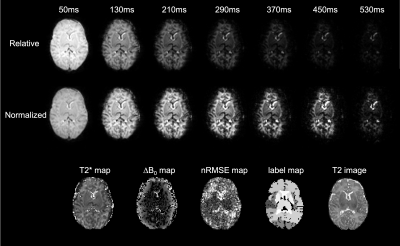
Top:
Neonatal T2* weighted slice acquired at increasing TE values using the proposed
method. Intensity scale in all images is fixed relative to the 1st echo (TE 50ms).
Middle: Intensity scale is normalized to each individual echo for the same data
as in the row above. Bottom: corresponding T2* map, through-slice susceptibility-induced
background gradient map, normalized RMSE map, segmented labels and the corresponding
T2 slice used for the proposed registration and segmentation method.
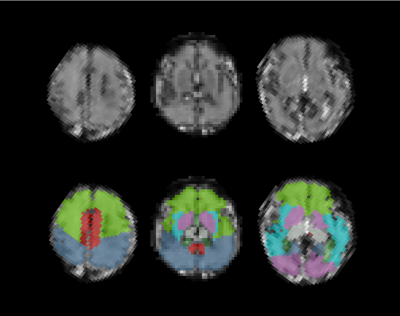
An example of the
automatic segmentation of a low resolution T2* weighted slice for a moving fetal
subject using the proposed framework. Labels were originally determined on a 3D
reconstructed high resolution T2 volume from the same subject with a gestational-age
matched atlas. Next an iterative registration scheme followed between the 3D reconstructed
T2 volume and the individual low resolution T2* slices. Finally, the
corresponding labels could be propagated to each T2* slice.
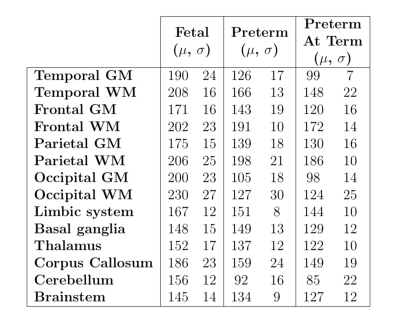
Mean
and standard deviation of T2* constant estimates (in ms) in 14 regions of
interest measured from the segmented labels as outlined in the proposed method.
The T2* values were corrected for susceptibility-induced background gradients
in neonatal subjects due to air-tissue interface. Outliers with high nRMSE were
removed. All T2* measurements were made in the acquired image space with no voxel
interpolation.
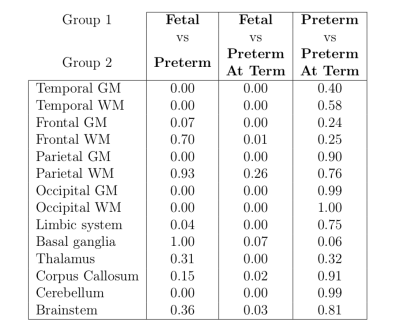
Pairwise comparisons
of T2* mean estimates between subject groups using a two tailed t-test. Significant
differences are found in cerebellum, limbic system and all cortical regions
except for frontal white matter and parietal white matter between fetal and preterm
subjects, indicating non-linear change in T2* values at the equivalent
gestational age. No significant differences are detected in any regions between
preterm and preterm at term subjects.
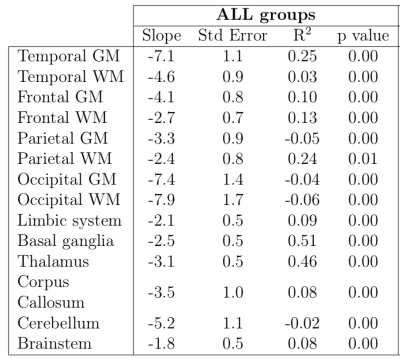
Linear correlation estimates
between T2* constants and gestational age. R2 and its p-value
estimates indicate the strength and significance of the correlation. Comparison
is made for all 3 subject groups combined. Only basal ganglia and thalamus
exhibit significant p-value and high R2 values, while the remaining
regions only show significant p-values. This suggests that a linear model is
not a good fit to the data in the remaining ROIs and the relationship may be more complex.