0094
Parietal GABA in children with Autism Spectrum Disorder and typically developing peers: distinct age-related changes1Brain and Mind Centre, Children's Hospital Westmead Clinical School, University of Sydney, Camperdown, Australia, 2Department of Radiology, University of Calgary, Calgary, AB, Canada, 3Hotchkiss Brain Institute, Calgary, AB, Canada, 4Alberta Children's Hospital Research Institute, Calgary, AB, Canada, 5Brain and Mind Centre, University of Sydney, Sydney, Australia
Synopsis
GABA, the mature brain’s primary inhibitory neurotransmitter, has been proposed to contribute to the development of Autism Spectrum Disorder (ASD) and the maintenance of ASD symptoms. Investigations have found reductions in GABA in children and adolescents with ASD. In the current study, GABA levels were measured using GABA-edited MEGA-PRESS in the left parietal lobe. The study compared 24 children with ASD and 35 typically developing (TD), aged 4-12 years. Increasing GABA concentration with age was found in the ASD participants but not in the TD cohort, suggesting a distinct pattern of GABA development in ASD within the parietal lobe.
Introduction
Alterations in the γ-aminobutyric acid (GABA) system have been proposed to contribute to the development of Autism Spectrum Disorder (ASD). GABA acts as the primary inhibitory neurotransmitter throughout life; providing a balance to excitatory glutamate to facilitate brain activity. Proton magnetic resonance spectroscopy (1H-MRS) allows for the measurement of GABA concentration in vivo. Reductions in GABA are proposed to result in an overly excitatory cortex and suggested to explain, or contribute to, symptoms of ASD and common comorbid conditions (e.g. epilepsy) [1]. In adults, it is established that GABA concentrations vary regionally [2]. In children and adolescents with ASD, there have been reductions found in GABA concentration in sensorimotor, frontal, temporal and cerebellar regions but not in occipital or prefrontal regions [3]. In adults with ASD, these group differences in GABA levels have not been detected; however, altered GABAergic transmission is hypothesized to persist [3].This study investigated the developmental trajectory of GABA, as measured using GABA-edited 1H-MRS in the left parietal lobe, a region key for social cognition and language. A cross-sectional design was used to compare children with ASD to their typically developing peers. We are specifically interested in the group differences and age-related effects on GABA concentration in order to better understand the differences in brain biology in ASD.
Methods
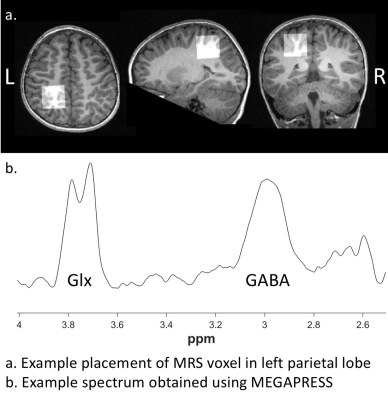
Twenty-four children with ASD (age: 9.11± 2.3, 4-12 years) and 35 typically developing children (age: 8.81 ± 2.26, 4-12 years) participated in the study. Magnetic Resonance Spectroscopy (MRS) was conducted on a 3-Tesla GE Discovery MR750 scanner using an eight-channel phased array head coil.Single voxel MEGA-PRESS data was acquired in the left parietal lobe for GABA quantification (TR = 1,800 ms; TE = 68 ms; editing pulses at 1.89ppm and 7.47 with a 14ms duration; 16 water averages and an 8-step phase cycle; number of averages = 256; number of points = 4096; spectral width = 5000 Hz; voxel size = 3x3x3 cm3). Positioning was guided by the T1-weighted image, maximising tissue content and avoiding skull. An example voxel placement is illustrated in Figure 1.
The MEGA-PRESS data was processed using Gannet [4], version 3.1. Briefly, this involves phase and frequency correction and frequency domain peak-fitting in order to estimate the concentration of GABA within the voxel of interest. The voxel was segmented using spm12 and the proportion of each tissue type was used for tissue correction, both to account for different relaxation properties of tissue types [5] and to adjust the measured signal based on the assumption that there is twice the concentration of GABA in grey matter compared to white matter the a-correction (i.e., a=0.5) [6].
Results
An ANCOVA was used to examine group differences in GABA concentration, with age included as a covariate to investigate its influence on GABA. There was a significant influence of diagnosis (p = 0.033), age (p = 0.005) and interaction of diagnosis and age (p = 0.029) on GABA concentration. Participants with ASD showed lower GABA concentrations at younger ages compared to TDC (B = -0.835, p = 0.033) and participants with ASD show a significant increase in GABA with age (B = 0.092, p = 0.029), whereas GABA levels in TD children do not change with age.There were no correlations between symptom severity measures (the Social Responsiveness Scale [SRS] and the Autism Diagnostic Observation Schedule [ADOS]) and GABA concentration.
Discussion
This cross-sectional study suggests a distinct trajectory of GABA development in children with ASD compared to their TD peers. Specifically, children with ASD showed age-related increases in GABA concentration while TD participants did not. Our data suggests GABA levels in younger children with ASD are lower when compared to TD but in older groups, this group difference does not persist. Our finding is consistent with the commonly reported GABA reductions in childhood in ASD as well as the lack of differences in GABA differences between ASD and TD groups in adulthood. In the current study, we found at age 9 the ASD group attained TD GABA levels. As regional variations in GABA are well established, we expect these age-related changes in GABA likely vary across the cortex.Conclusion
This study illustrates an age-related shift in children with ASD not seen in their TD counterparts. This finding offers a potential explanation for why reductions in GABA are seen in children and adolescents with ASD but not in adult populations.Acknowledgements
We acknowledge a BUPA Foundation Grant and an Endeavour Foundation Grant. We also acknowledge Project Grants (1043664 and 1125449) to Adam J. Guastella, a NHMRC senior principal research fellowship (APP1136259) to Ian B. Hickie.
We wish to thank the staff at i-Med Radiology Camperdown, especially Domenic Soligo, Pia Wilkstrom and Dr. Lynette Masters, for their assistance in data acquisition.
References
1. Cellot, G. and E. Cherubini, GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr, 2014. 2(70): p. 70.
2. Grewal, M., et al., GABA quantitation using MEGA-PRESS: Regional and hemispheric differences. J Magn Reson Imaging, 2016. 44(6): p. 1619-1623.
3. Ajram, L.A., et al., The contribution of [1H] magnetic resonance spectroscopy to the study of excitation-inhibition in autism. Prog Neuropsychopharmacol Biol Psychiatry, 2019. 89: p. 236-244.
4. Edden, R.A., et al., Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging, 2014. 40(6): p. 1445-52.
5. Gasparovic, C., et al., Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine, 2006. 55(6): p. 1219-1226.
6. Harris, A.D., N.A.J. Puts, and R.A.E. Edden, Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. Journal of Magnetic Resonance Imaging, 2015. 42(5): p. 1431-1440.