0091
Time-dependent Diffusion MRI of Pediatric Brain tumor at 3T1Children's Hospital, Zhejiang University School of Medicine, Hangzhou, China, 2Nemours AI duPont Hospital for Children, Wilmington, DE, United States, 3Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China
Synopsis
Diffusion-time dependent diffusion MRI has shown potential in probing tumor microstructure. This study investigated the feasibility of time-dependent dMRI to map brain tumor microstructure in a pediatric population at 3T. Oscillating and pulsed gradient dMRI was performed to access a series of diffusion times and b-values, and the data were fitted with the IMPULSED model to estimate cell diameter, intracellular fraction, and diffusivity metrics. In a pilot study of 17 pediatric brain tumor patients, all high-grade tumors showed higher intracellular fraction and cellularity than the low-grade ones, while the cell diameter showed differentiation among different types of high-grade tumors.
Introduction
Recent progress on diffusion-time (td) dependent diffusion MRI 1,2 offers new insights into tissue microstructure. Particularly, the technique has unique advantages in characterizing tumor microstructure, as reported in several preclinical studies 3-6. Despite the challenges to achieve short td on clinical scanners, recently, the feasibility of td-dependent dMRI in clinical tumor applications 7-11 has also been explored. In this study, we investigated the utility of td-dependent dMRI based microstructural mapping in pediatric brain tumor patients, using pulsed and oscillating gradients on a clinical 3T system.Methods
Seventeen pediatric brain tumor patients (aged 8 months to 14 years old, 8 males and 9 females) were enrolled in the study with IRB approval and parental consent. An oscillating gradient dMRI (OG-dMRI) sequence with trapezoid-cosine gradients was implemented on a 3T Phillips Achieva scanner (maximum gradient =80 mT/m). The OG-dMRI was performed at oscillating frequencies of 50Hz (b=0.37 ms/µm2), 33Hz (b=0.5 ms/µm2), and 17Hz (b=0.5/1/2 ms/µm2), 4 diffusion directions, TE/TR = 168/3000 ms, FOV=180×180 mm, in-plane resolution = 1.4×1.4 mm, 3 slices with a slice thickness of 8 mm. Pulse gradient dMRI (PG-dMRI) was performed with σ/Δ = 60/82.8 ms (b=0.5/1/2 ms/µm2), and the other parameters were kept the same as OG-dMRI. The total protocol was under 5min. Routine T1-weighted images before and after Gd-enhancement, T2-weighted, FLAIR, and routine DWI (b=0.6 ms/µm2) were also obtained.The PG- and OG-dMRI data were fitted using the imaging microstructural parameters using limited spectrally edited diffusion (IMPULSED) method 3, assuming a two-compartment model with impermeable cells. The fitting was performed using least square curve fitting in MATLAB, and the fitting was repeated 100 times with randomized initialization to avoid local minimum. Cell diameter (d), intracellular fraction (fin) and extracellular diffusivity (Dex) were estimated, while the intracellular diffusivity (Din) was fixed at 1 µm2/ms since the fitting is insensitive to the choice of Din 7. The cellularity was measured as fin/d. The regions of interest (ROIs) were defined in the solid region of the tumor based on b0 images.
Results
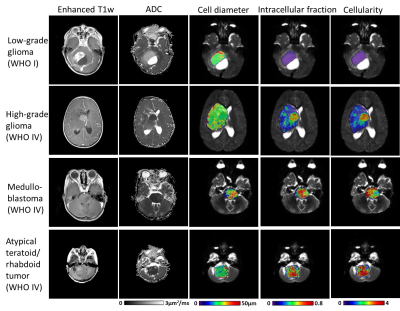
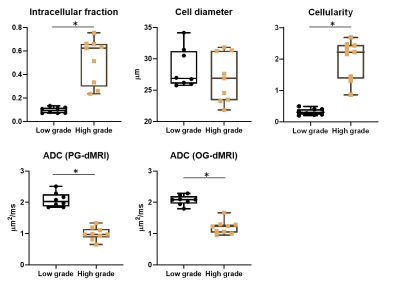
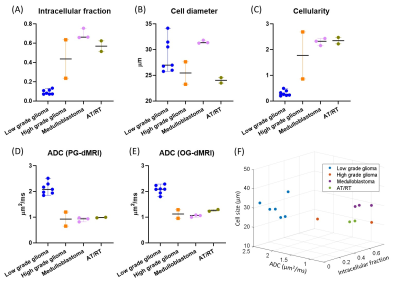
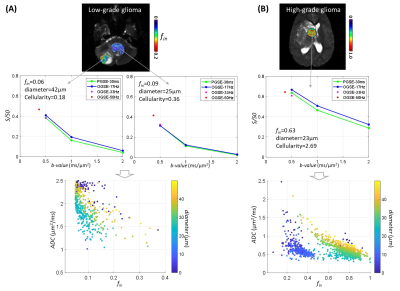
Based on the pathological examination, among the 17 patients, seven had low-grade glioma (WHO I and II), two had high-grade glioma (WHO IV), three had medulloblastoma (WHO IV), two had atypical teratoid/rhabdoid tumor (AT/RT, WHO IV), along with the other types of tumors (n=1 per category). Figure 1 demonstrated that microstructural mapping results in four types of pediatric tumors, along with the apparent diffusivity coefficient (ADC) maps from routine DWI and Gd-enhanced T1w images. It is evident that the fin and cellularity were much higher in high-grade glioma, medulloblastoma, and AT/RT, compared with that in low-grade glioma, while the Gd-enhanced images could not distinguish high-grade from low-grade tumors in these four cases. The low and high-grade tumors can be separated based on fin (5.4 folds difference) and cellularity (6.3 folds difference) (Figure 2). The ADC values from individual PGSE and OGSE (33Hz) scans also showed significant differences between low and high-grades with relatively smaller effective sizes (2.1 and 1.7 fold differences, respectively).When looking into the specific tumor types, we noticed that the medulloblastoma showed a larger cell size compared to the other types of high-grade tumors (Figure 3). By examining the dMRI signals at different td and b-values (Figure 4), it is observed that the td-dependence was higher in high-grade tumors than the low-grade ones. In addition, we found a negative relationship between ADC (PG-dMRI) and fin based on the scatter plots of individual voxels (Figure 4), which may explain the diagnostic performance of both metrics.
Discussion and Conclusion
This pilot study demonstrated that td-dependent dMRI based microstructural mapping of brain tumor was feasible on clinical systems, and it is capable of tumor grading for typical types of pediatric tumors. The microstructural information, such as cell size and cellularity, may provide valuable information about the tumor pathology, e.g., the low fin in low-grade glioma (lower than normal tissue) indicates loose and less cellular micro-environment with cyst formations. The added information may also be useful in differentiating different tumor types, e.g., the medulloblastoma (a type of embryonic tumor) showed a larger cell size compared to other high-grade tumors. Histological evidence and more samples are needed to support these findings.Another limitation lies in that the IMPULSED model, like the other existing models 4,5, assumed a simple two-compartment configuration, and permeability was not considered. Therefore, the model may not be appropriate for normal brain tissue, resulting in underestimation of fin and unstable fitting of cell size in non-tumor regions. Nevertheless, the model requires relatively small q-t space samples and provided useful microstructural characterization of the tumor. Given the relatively short scan time, the current imaging protocol can be readily incorporated into clinical practice.
Acknowledgements
This work was supported by the Natural Science Foundation of China (61801424, 81971606, and 91859201) and the Ministry of Science and Technology of the People’s Republic of China (2018YFE0114600).References
1. Stepisnik J. Time-Dependent Self-Diffusion by Nmr Spin-Echo. Physica B 1993;183(4):343-350.
2. Gore JC, Xu JZ, Colvin DC, Yankeelov TE, Parsons EC, Does MD. Characterization of tissue structure at varying length scales using temporal diffusion spectroscopy. NMR in biomedicine 2010;23(7):745-756.
3. Jiang XY, Li H, Xie JP, Zhao P, Gore JC, Xu JZ. Quantification of cell size using temporal diffusion spectroscopy. Magn Reson Med 2016;75(3):1076-1085.
4. Reynaud O, Winters KV, Hoang DM, Wadghiri YZ, Novikov DS, Kim SG. Pulsed and oscillating gradient MRI for assessment of cell size and extracellular space (POMACE) in mouse gliomas. NMR in biomedicine 2016;29(10):1350-1363.
5. Panagiotaki E, Walker-Samuel S, Siow B, Johnson SP, Rajkumar V, Pedley RB, Lythgoe MF, Alexander DC. Noninvasive Quantification of Solid Tumor Microstructure Using VERDICT MRI. Cancer Res 2014;74(7):1902-1912.
6. Colvin DC, Yankeelov TE, Does MD, Yue Z, Quarles C, Gore JC. New insights into tumor microstructure using temporal diffusion spectroscopy. Cancer Res 2008;68(14):5941-5947.
7. Xu J, Jiang X, Li H, Arlinghaus LR, McKinley ET, Devan SP, Hardy BM, Xie J, Kang H, Chakravarthy AB, Gore JC. Magnetic resonance imaging of mean cell size in human breast tumors. arXiv 2019;arXiv:1905.07818.
8. Lemberskiy G, Rosenkrantz AB, Veraart J, Taneja SS, Novikov DS, Fieremans E. Time-Dependent Diffusion in Prostate Cancer. Invest Radiol 2017;52(7):405-411.
9. Lima M, Kataoka M, Honda M, Ohno AK, Ota R, Ohashi A, Urushibata Y, Feiweier T, Toi M, Togashi K. Time-Varying Diffusion Patterns in Breast Cancer Linked to Prognostic Factors. 2019; Montreal, QC, Canada.
10. Lemberskiy G, Baete S, Novikov D, Fieremans E, Zan E, Hu K, Kim S. Diffusion time-dependence of diffusivity and kurtosis in locally advanced head and neck squamous cell carcinoma before and after chemo-radiation therapy. 2019; Montreal, QC, Canada.
11. Reynaud O. Time-Dependent Diffusion MRI in Cancer: Tissue Modeling and Applications. Front Phys 2017;5.
Figures