0089
Improved data quality and reduced costs by slice localization integrated MRI monitoring
Yao Sui1,2, Onur Afacan1,2, Ali Gholipour1,2, and Simon Keith Warfield1,2
1Harvard Medical School, Boston, MA, United States, 2Boston Children's Hospital, Boston, MA, United States
1Harvard Medical School, Boston, MA, United States, 2Boston Children's Hospital, Boston, MA, United States
Synopsis
Motion monitoring has shown helpful in MRI, particularly in long acquisitions such as 2D echo-planar imaging for fMRI. The most widely-used motion monitoring for fMRI relies on volume-to-volume registration (VVR). However, motion happens at the slice level, and VVR is insufficiently sensitive to intra-volume motion. In this work, we present the first slice-by-slice self-navigated motion monitoring system for MRI via a real-time slice-to-volume registration (SVR) algorithm. Extensive experiments demonstrated that our approach provides accurate motion measurements, and allows adaptive acquisition that ensures sufficient amount of data, while not acquiring data in excess, leading to improved data quality and reduced costs.
Introduction
Head motion adversely affects MRI data quality, leading to distorted images and increased cost due to the need for repeated scanning1, in particular in longer acquisitions such as fMRI. Research has shown that even sub-millimeter head movements systematically degrade the accuracy of fMRI analyses2. To ensure sufficient data for analysis, a typical strategy, in the face of unknown motion-induced data loss, is to acquire additional data3. Although such a strategy increases likelihood of collecting sufficient data, it is highly inefficient, significantly increases imaging time and costs, and ultimately does not guarantee acquiring data of sufficient quality in a cohort such as infants, toddlers, and young children4. The increased imaging time due to motion has been estimated to cost clinical and research studies $1.4B per year in the US5. Therefore, motion monitoring in fMRI should be done quantitatively (scanning-to-criterion) and online (real-time) to ensure sufficient data is acquired, and avoid unnecessary long scans that waste time, are costly, and are burdensome for patients. Real-time motion monitoring in fMRI has shown to be helpful6 and has been investigated in various ways. Methods with external hardware7 and navigators8 are platform-dependent, complicated to setup, increase costs, and may raise safety concerns in certain patient populations. A recent technique FIRMM6 is a self-navigated method for motion monitoring during fMRI with real-time performance. However, as illustrated in Fig. 1, FIRMM uses VVR to infer motion parameters, resulting in insufficiently accurate motion measurements and large temporal delays. In this work, we report the first development and evaluation of a real-time slice-by-slice motion monitoring algorithm for brain MRI, named Slice Localization Integrated MRI Monitoring (SLIMM).Material and Methods
We employed an EPI-based acquisition for fMRI with an interleaved and simultaneous multi-slice scheme. To achieve real-time performance, our technique extracts a set of local image features and incorporates a feature selection scheme to create a similarity metric for SVR. Accelerated optimization is achieved through finite difference computation of the similarity gradients over transformation parameters within a Levenberg-Marquardt optimization framework. We developed an auto-calibration algorithm via SVR to obtain a volume without intra-volume motion as the reference. As fMRI slices are acquired, we register each slice to the reference volume by our real-time SVR algorithm over a rigid-body motion model. Motion is thus measured by the change in the position of the acquired slice before and after registration.We acquired the fMRI data from 7 volunteers and 2 patients with a 3T Siemens MR scanner, using 2D gradient-echo EPI sequences with 96 measurements, and interleave and simultaneous multi-slice factors of 2 and 2, respectively, TR/TE=3000/30ms, slice thickness=3mm, #slices=36, matrix size=64x64. For volunteer scans, we used an electromagnetic9 or a Kineticor10 optical motion tracking sensor to record head motion as a reference. We implemented a VVR-based method as the baseline, denoted by VVR-LM.
Results
Our approach processed 27.7 slices per second on average on a workstation with 20 cores of Intel Xeon CPU@2.2GHz. Fig. 2 shows the motion measurements in three rotation parameters in the case of continuous motion. Our method, SLIMM closely matched the reference motion pattern measured by a Kineticor motion tracking sensor, and offered sub-voxel slice-level accuracy, in particular for fast motion. Fig. 3 shows the distributions of the tSNR scores obtained from VVR-LM and SLIMM on all the volunteer data. Our method, SLIMM, substantially improved the tSNR. Fig. 4 shows the percentage of volumes with different motion amounts measured by framewise displacement over the original patient data and the corrected data through VVR-LM and SLIMM. SLIMM resulted in over 82% volumes without motion (displacement<0.005mm). In contrast, VVR-LM led to approximately only 50% volumes without motion. Fig. 5 shows that for about 80% of the subjects in the Human Brain Network11 dataset, auto-calibration was complete after acquiring the second volume, and the first volume was selected as reference, and for 99.5% of the subjects the auto-calibration time was less than 30 seconds according to our fMRI protocol.Discussion
We have demonstrated slice-by-slice motion monitoring improved data quality and reduced costs. We have shown that the auto-calibration worked well for the majority of subjects in a large pediatric cohort, and in a few cases with significant motion, it took relatively long time. It indicates that we cannot just rely on a long acquisition protocol for the entire cohort, hoping to get sufficient data for all cases. Without real-time motion monitoring, we may end up scanning a large number of subjects for unnecessary long scan time, and yet not be successful in acquiring useful data for some subjects in the cohort. With real-time motion monitoring and an adaptive strategy for extending the length of fMRI scans, until a desirable number of “motion-free” periods are collected, we can ensure that an fMRI protocol runs efficiently for a cohort of patients and also for an individual patient scanned in a clinical setting. Therefore, real-time motion monitoring substantially reduces average scan times and associated costs. Our approach (SLIMM) is a cost-efficient and safe, self-navigated, fast, slice-level motion monitoring system, that does not require any external hardware attachment or pulse sequence modification, therefore it can be safely and easily used with different fMRI paradigms for pediatric and non-cooperative patients.Acknowledgements
Research reported in this publication was supported in part by the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Neurological Disorders and Stroke, and the National Institute of Mental Health of the National Institutes of Health (NIH) under Award Numbers R01EB019483, R01EB018988, R01NS079788, R01NS106030, and R44MH086984; and by a Technological Innovations in Neuroscience Award from the McKnight Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the McKnight Foundation.References
- M. Zaitsev, J. Maclaren, and M. Herbst, “Motion artifacts in MRI: a complex problem with many partial solutions,” Journal of Magnetic Resonance Imaging, vol. 42, no. 4, pp. 887-901, 2015.
- J. D. Power, K. A. Barnes, A. Z. Snyder, B. L. Schlaggar, and S. E. Petersen, “Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion,” Neuroimage, vol. 59, no. 3, pp.2142-2154, 2012.
- R. Ciric, D. Wolf, J. Power, D. Roalf, G. Baum, K. Ruparel, R. Shinohara, M. Elliott, S. Eickhoff, C. Davatzikos, R. Gur, R. Gur, D. Bassett, and T. Stterthwaite, “Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity,” Neuroimage, vol. 154, pp. 174-187, 2017.
- O. Afacan, B. Erem, D. P. Roby, N. Roth, A. Roth, S. P. Prabhu, and S. K. Warfield, “Evaluation of motion and its effect on brain magnetic resonance image quality in children,” Pediatric Radiology, vol. 46, no. 12, pp. 1728-1735, 2016.
- J. B. Andre, B. W. Bresnahan, M. Mossa-Basha, M. N. Hoff, C. PatrickSmith, Y. Anzai, and W. A. Cohen, “Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations,” Journal of the American College of Radiology, vol. 12, no. 7, pp. 689-695, 2015.
- N. Dosenbach, J. Koller, E. Earl, O. Miranda-Dominguez, R. Klein, A. Van, A. Snyder, B. Nagel, J. Nigg, A. Nguyen, V. Wesevich, D. Greene, and D. Fair, “Real-time motion analytics during brain MRI improve data quality and reduce costs,” Neuroimage, vol. 161, pp. 80-93, 2017.
- J. Schulz, T. Siegert, P.-L. Bazin, J. Maclaren, M. Herbst, M. Zaitsev, and R. Turner, “Prospective slice-by-slice motion correction reduces false positive activations in fMRI with task-correlated motion,” NeuroImage, vol. 84, pp. 124-132, 2014.
- D. C. Hoinkiss and D. A. Porter, “Prospective motion correction in 2D multishot MRI using EPI navigators and multislice-to-volume image registration,” Magnetic Resonance in Medicine, vol. 78, no. 6, pp. 2127-2135, 2017.
- O. Afacan, T. Wallace, and S. K. Warfield, “Retrospective correction of head motion using measurements from an electromagnetic tracker,” Magnetic Resonance in Medicine, 2019.
- M. Zaitsev, C. Dold, G. G.Sakas, J. Hennig, and O. Speck, “Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system,” NeuroImage, vol. 31, no. 3, pp. 1038-1050, 2006.
- L. Alexander et al., “An open resource for transdiagnostic research in pediatric mental health and learning disorders,” Scientific Data, vol. 4, 2017.
Figures
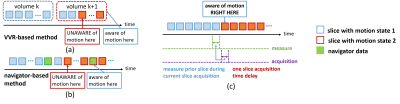
Illustration of image alignment-based motion measurement methods. (a) Volume-to-volume registration (VVR) based method. The techniques in this category have large temporal delays and insufficient accuracy in measuring intra-volume motion. (b) Navigator image-based method. This method requires to modify the imaging protocols and is thus platform-dependent. (c) Slice-to-volume registration (SVR) based method. The techniques in this category have the minimum temporal delay and highly accurate motion measurements in slice-by-slice acquisitions.
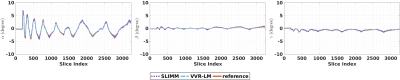
Motion measurements in terms of three rotation parameters on the optical motion tracking data set. Our method (SLIMM) closely matched the reference motion pattern measured by a Kineticor optical motion tracking sensor. The overall means and standard deviations of the registration errors in terms of slicewise displacement were: VVR-LM 0.7672±0.9380, SLIMM 0.3243±0.2248 (mm), and in the period of fast motion (data points in the range of [200, 300]): VVR-LM 3.1171±2.2389, SLIMM 0.4379±0.3718 (mm). Our method offered sub-voxel slice-level accuracy in this difficult task.
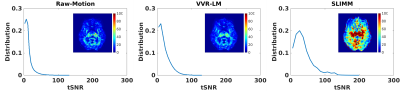
Distributions of the tSNRs over data of all volunteers calculated by our and the VVR-based methods compared to Raw-Motion data acquired with in-scanner motion. The tSNR map calculated from the retrospectively corrected fMRI data by each method is also shown for an axial slice of a representative volunteer. The results show that our method, SLIMM, substantially improved the tSNR of the Raw-Motion data and outperformed VVR-LM. While tSNR is lost naturally due to motion in fMRI, our method was able to recover a large portion of tSNR despite the subject motion that occurred during the scans.
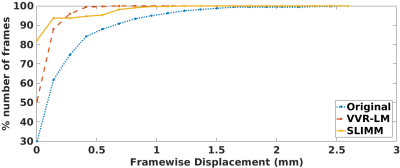
The percentage of fMRI volumes (frames) with different motion amounts in terms of framewise displacement over the original patient data and the corrected patient data through the motion measurements obtained from VVR-LM and SLIMM respectively. SLIMM resulted in over 82% volumes without motion (displacement<0.005mm). In contrast, VVR-LM led to approximately only 50% volumes without motion. The average tSNRs on all voxels of tSNR volumes on the patient data set were: original data 44.06, VVR-LM 48.10, and SLIMM 55.05.
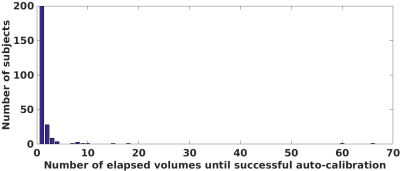
Distribution of the number of subjects over number of elapsed volumes until auto-calibration was successful with a threshold of 1/4 of the slice thickness on the Human Brain Network dataset containing the resting-sate fMRI data acquired from 251 subjects. This analysis shows a heavy-tailed distribution with concentration around 1. It indicates that for the majority of (~200) subjects, the first volume was chosen as the reference volume; whereas for a few subjects, that apparently moved continuously, it took between 15 to 70 volumes before a motion-free reference volume was obtained.