0086
Free-Breathing Volumetric Liver R2* Quantification in Pediatric Patients Using 3D Self-Gating Motion-Compensated Stack-of-Radial MRI1MR R&D Collaborations, Siemens Healthcare, Los Angeles, CA, United States, 2Department of Radiology, Nationwide Children's Hospital, Columbus, OH, United States, 3Department of Radiological Sciences, University of California Los Angeles, Los Angeles, CA, United States, 4Department of Physics and Biology in Medicine, University of California Los Angeles, Los Angeles, CA, United States, 5MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 6MR R&D Collaborations, Siemens Healthcare, Austin, TX, United States
Synopsis
Liver fat and iron quantification is of growing interest. However, it is challenging and sometimes impossible to perform breath-hold MRI acquisitions in children. Using a breath-hold 3D Cartesian method as reference, a self-gating free-breathing 3D stack-of-radial liver R2* quantification technique was evaluated. Results showed that the free-breathing stack-of-radial technique accurately quantified fat even without self-gating, while free-breathing R2* quantification had biases caused by respiratory motion and self-gating was necessary for accurate R2* quantification in pediatric subjects. This technique has potential for accurate and efficient free-breathing quantification of both liver fat and iron in pediatric patients.
INTRODUCTION
There is a growing interest to quantify liver fat and iron in children.1 For liver proton density fat fraction (PDFF) and R2* quantification, breath-hold three-dimensional (3D) multi-echo Cartesian gradient-echo (GRE) MRI has been clinically utilized in adult patients.2-4 However, it is challenging and sometimes impossible to perform breath-hold MRI in children.For free-breathing acquisitions, 3D stack-of-radial imaging has been shown to provide accurate liver PDFF quantification after gradient delay error correction in adult and pediatric patients.5-7 A recent study revealed impacts of respiratory motion on free-breathing R2* quantification using the 3D stack-of-radial imaging approach, proposed a self-gating solution for compensation, and validated the method in adult subjects.8 The purpose of this study was to perform a preliminary evaluation of the free-breathing stack-of-radial liver R2* quantification technique8 in pediatric patients, using a breath-hold 3D Cartesian method4 as reference.
METHODS
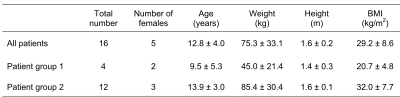
Imaging Sequences and ProtocolsThis study was approved by the Institutional Review Board with written informed consent obtained from parents/guardians. In-vivo liver imaging data were acquired in 16 pediatric patients with suspected liver disease referred for 3T MRI (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). Demographic information of the patients is in Table 1.
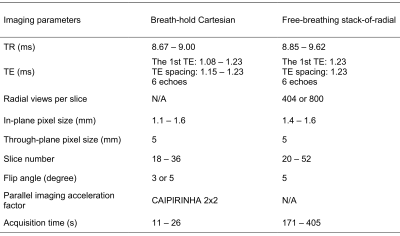
A multi-echo GRE stack-of-radial prototype sequence8 was used for free-breathing data acquisition with golden-angle ordering9,10 and gradient delay calibration5-7 (parameters in Table 2). The coil channel used for eventually extracting the respiratory self-gating signal from the center of k-space was determined by an automatic channel selection algorithm12, or manually selected when necessary. The self-gating signal was used to accept data acquired near end-expiration. For reference, a breath-hold 3D Cartesian GRE prototype sequence4 was performed with parameters in Table 2. The acquisition time of acquisitions varied depending on differences in protocol settings, e.g. partitions and radial views. Images were reconstructed inline, and to allow the most flexibility, raw data were saved for retrospective analysis of reconstruction parameters.
Data Processing and Statistical Analysis
To evaluate the effect of self-gating, the 3D stack-of-radial data were reconstructed offline without self-gating and with a 40% self-gating acceptance rate, respectively,8, 11,12 after the gradient delay correction.5-8 PDFF and R2* quantification of both the reference breath-hold Cartesian and the free-breathing stack-of-radial acquisitions were performed using the same algorithm.4
Two regions of interest (ROIs) on the left and right lobes of the liver, respectively, were manually drawn on the R2* maps by an experienced MR researcher. Large hepatic vessels and severe imaging artifacts were avoided when drawing ROIs. For each subject, R2* values of these two ROIs were recorded as mean ± standard deviation (SD), and averaged. The PDFF values of the same ROIs were also measured and averaged for each subject. Bland-Altman analysis was performed to determine the mean difference (MD) and limits of agreement (LoA = MD ± 1.96×SD) between the free-breathing and breath-hold methods.
RESULTS
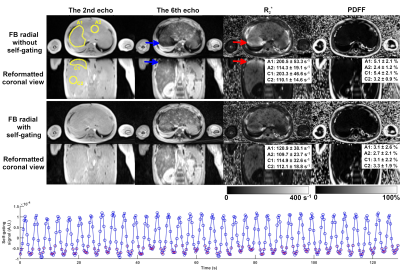
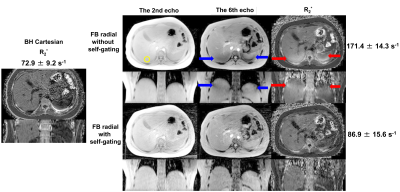
Example images and R2* maps of a 2-year-old pediatric patient are shown in Figure 1. Strong signal attenuation (blue arrows in the upper two rows) was observed on the late-echo images (the 6th echo as an example) without self-gating. This attenuation led to artificially elevated values in the R2* maps (red arrows in the upper two rows). This was eliminated by self-gating motion compensation.Another example of an 11-year-old pediatric patient is shown in Figure 2. Similarly, signal attenuation in late-echo images and artificially elevated R2* values were observed in free-breathing stack-of-radial acquisitions without self-gating (blue and red arrows in the upper two rows), and were largely improved by self-gating, compared to the reference R2* map of a breath-hold Cartesian acquisition.
The free-breathing stack-of-radial scans were completed in all subjects. However, 4 subjects suffered from serious respiratory artifacts or problematic acquisition of the breath-hold Cartesian scans and were excluded from the statistical analysis (Table 1).
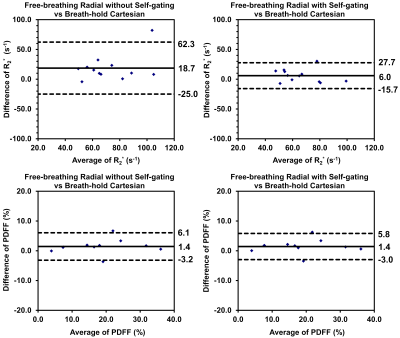
Bland-Altman plots are shown in Figure 3. For R2*, compared to the breath-hold Cartesian reference, free-breathing stack-of-radial without self-gating showed MD of 18.7 s-1 as well as relatively wide LoA of -25.0 to 62.3 s-1. In contrast, free-breathing stack-of-radial with self-gating showed an improved MD of 6.0 s-1, and LoA of -15.7 to 27.7 s-1. For PDFF, no obvious biases of MD values were observed between non-self-gated and self-gated reconstruction, and the LoA ranges were similar for both methods.
DISCUSSION
In this work, a free-breathing R2* quantification technique based on 3D stack-of-radial imaging with self-gating was evaluated in pediatric patients. For the protocols and patients evaluated in this study, free-breathing R2* quantification suffered from a bias introduced by respiratory motion, if not compensated. A 3D stack-of-radial imaging approach with self-gating was proposed for respiratory motion compensation, and the in-vivo results and analyses demonstrated the accuracy of the proposed method compared to reference results of breath-hold Cartesian MRI. The observation was consistent with what was reported for adult subjects.8 Additional studies are warranted to further evaluate this approach in a larger number of pediatric patients.CONCLUSION
The proposed free-breathing stack-of-radial R2* quantification technique with self-gating has potential to accurately and efficiently evaluate liver iron content in pediatric patients. With no burden of breath-hold required for data acquisition, the proposed method may be an attractive option to measure liver iron in children.Acknowledgements
This study was supported in part by Siemens Medical Solutions USA, Inc. and the Department of Radiological Sciences at UCLA.References
1. Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:13-24.
2. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water‐fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122-1134.
3. Hernando D, Levin YS, Sirlin CB, Reeder SB. Quantification of liver iron with MRI: state of the art and remaining challenges. J Magn Reson Imaging. 2014;40:1003-1021.
4. Zhong X, Nickel MD, Kannengiesser SAR, Dale BM, Kiefer B, Bashir MR. Liver fat quantification using a multi‐step adaptive fitting approach with multi‐echo GRE imaging. Magn Reson Med. 2014;72:1353-1365.
5. Peters DC, Derbyshire JA, McVeigh ER. Centering the projection reconstruction trajectory: reducing gradient delay errors. Magn Reson Med. 2003;50:1-6.
6. Armstrong T, Dregely I, Stemmer A, et al. Free-breathing liver fat quantification using a multiecho 3D stack-of-radial technique. Magn Reson Med. 2018;79:370-382.
7. Armstrong T, Ly KV, Murthy S, et al. Free-breathing quantification of hepatic fat in healthy children and children with nonalcoholic fatty liver disease using a multi-echo 3-D stack-of-radial MRI technique. Pediatr Radiol. 2018;48:941-953.
8. Zhong X, Armstrong T, Nickel MD, et al. Effect of respiratory motion on free‐breathing 3D stack‐of‐radial liver R2* relaxometry and improved quantification accuracy using self-gating. Magn Reson Med. 2019. In press.
9. Block KT, Chandarana H, Milla S, et al. Towards routine clinical use of radial stack-of-stars 3D gradient-echo sequences for reducing motion sensitivity. J Korean Soc Magn Reson Med. 2014;18:87-106.
10. Fujinaga Y, Kitou Y, Ohya A, et al. Advantages of radial volumetric breath-hold examination (VIBE) with k-space weighted image contrast reconstruction (KWIC) over Cartesian VIBE in liver imaging of volunteers simulating inadequate or no breath-holding ability. Eur Radiol. 2016;26:2790-2797.
11. Grimm R, Block KT, Hutter J, et al. Self-gating reconstructions of motion and perfusion for free-breathing T1-weighted DCE-MRI of the thorax using 3D stack-of-stars GRE imaging. In Proceedings of the 20th Annual Meeting of ISMRM, Melbourne, Australia, 2012. p. 598.
12. Grimm R, Bauer S, Kiefer B, Hornegger J, Block T. Optimal channel selection for respiratory self-gating signals. In Proceedings of the 21st Annual Meeting of ISMRM, Salt Lake City, UT, 2013. p. 3749.
13. Breuer FA, Blaimer M, Mueller MF, et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med. 2006;55:549-556.
Figures