0083
In vivo evaluation of white matter abnormalities in children with DMD using diffusion MRI1Nueroimaging and Interventional Radiology, National Institute of Mental Health and, Bangalore, India, 2Neurology, National Institute of Mental Health and, Bangalore, India, 33Symbiosis Centre for Medical Image Analysis, Symbiosis International University, Pune, India, 4Neuroimaging and Interventional Radiology, National Institute of Mental Health and, Bangalore, India, 5Symbiosis Centre for Medical Image Analysis, Symbiosis International University, Pune, India
Synopsis
Duchenne muscular dystrophy (DMD), a genetically inherited X-linked neuromuscular disorder characterised by progressive muscle weakness and significant non-motor manifestations like poor IQ, and neuropsychiatric illnesses. In this study, we evaluate white matter (WM) abnormalities in DMD patients using diffusion tensor imaging (DTI). We observed widespread WM changes in DMD patients and the presence of distal mutation was associated with poor clinical and neuropsychological profile with severe and spatially more WM abnormalities.
Introduction: Duchenne muscular dystrophy (DMD) is characterized by mutations in the DMD gene results in absent/non-functional muscle dystrophin protein leading to severe progressive muscle weakness1 along with significant non-motor manifestations like poor IQ, reading difficulties and increased prevalence of neuropsychiatric illnesses2–4. The DMD gene contains multiple independent tissue-specific promoters, producing several isoforms named according to their length and splicing patterns. The isoform Dp427m is predominantly expressed in muscles and plays a role in providing structural integrity to muscle fibers while Dp427c is expressed in cortex and hippocampus, Dp427p in Purkinje cells and shorter Dp260 and Dp116 isoforms primarily are expressed in the retina and peripheral nerve5-6. This current work aims to evaluate the brain abnormalities comprehensively in a larger cohort of DMD children and to further investigate the differences in WM abnormalities in two major subtypes based on Dp140 expression using DTI. We hypothesize that patients with retained Dp140 (Dp140+) expression will have relatively preserved WM as compared to patients with loss of Dp140 (Dp140-) isoform.
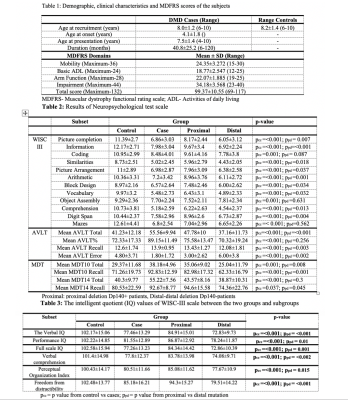
Material & Methods: We evaluated neuropsychological abnormalities and WM microstructural integrity in DMD patients (N=60; proximal mutation=39 and distal mutation=21), and controls (N=40). DMD patients were confirmed to have deletions by MLPA test7.
Neuropsychological Assessments were performed to assess the functionality, IQ, auditory verbal learning memory and WISC-III to assess Verbal, Performance, and Full-scale IQ, Verbal Comprehension, Perceptual Organization Index in all the children.
MRI: MRI were obtained on 3T Philips Achieva using 32-channel head-coil. A High-resolution 3D TFE T1W-images were acquired (TR/TE=9.8/4.6ms, and spatial-resolution=1x1x1mm). Single-shot spin-echo echo-planar DTI sequence with following parameters: TR/TE=5000/=65ms; resolution=2.0x2.0x2.0mm; non-coplanar diffusion directions=15, b value=0 and 1000s/mm2; with 2-repetitions.
Data processing and analysis: Diffusion data analysis was carried out using FMRIB Software Library tools (www.fmrib.ox.ac.uk/fsl) version- 5.0.11. WM integrity was examined using whole-brain TBSS and Multiple WM ROIs were defined using JHU-WM atlas, which is a probabilistic atlas generated by mapping DTI data of healthy subjects to a template image. The mean diffusion metric values of each ROI for each subject were extracted for atlas-based analysis. DMD subgroups based on deletion location were grouped into proximal (Dp 140+) and distal (Dp 140-). Neuropsychological scores and DTI metrics were compared between patients and controls and also between DMD subgroups.
Statistical Analysis: A comparison was carried using an independent sample t-test/Wilcoxon signed-rank test based on normality. For the group analysis on the WM tracts between two groups, ANOVA was employed to analyze all the mean diffusion metric values followed by the pairwise comparison with Bonferroni correction for multiple comparisons. Pearson’s correlation coefficient was computed, and the significance threshold was maintained at p-value<0.01. All the data were analyzed using SPSS-21.
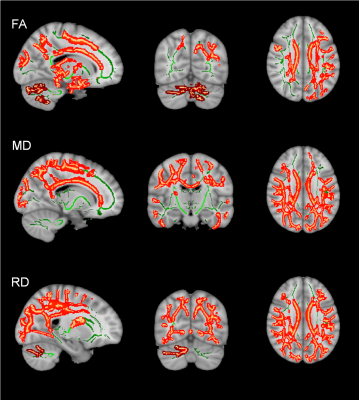
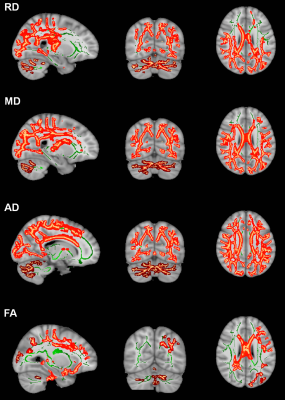
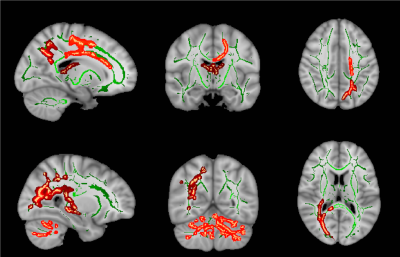
Results: The mean age of DMD and controls were 8.0±1.2years and 8.2±1.4years, respectively. Mean age at disease onset in DMD subgroup was 4.1±1.8years and mean illness duration was 40.8±25.2months (Table 1). We observed a significant difference in neuropsychological scores between DMD children with proximal and distal gene mutations and severe impairments noted in distal mutation (p<0.05) (Table 2 & 3). In patients with the proximal mutation, only localized FA changes were seen involving corpus-callosum, parietal WM, and Fornix. The distal subgroup showed widespread reduced FA and increased diffusivity in WM (Fig. 1). Compared to DMD children with proximal mutation, distal mutation showed increased axial diffusivity in various WM regions. No significant correlation was noted between clinical and neuropsychological scores with diffusion metrics (Fig. 2 and 3).
Discussion: In this present study, we have reported microstructural changes within DMD subgroup (i.e. proximal and distal mutation) using DTI. The current study revealed impaired IQ and neuropsychological abnormalities along with brain abnormalities, which were more severe in distal mutation Dp140-subgroup, and these findings are inlined with the previous studies2-4. We also observed widespread WM changes in DMD patients and the presence of the distal mutation was associated with poor clinical and neuropsychological profile with severe and spatially more WM abnormalities3, 8-11. Our DTI findings demonstrate widespread WM alterations involving both supratentorial and infratentorial WM12, 13. Between-group comparison of proximal and distal mutations with healthy controls using TBSS revealed significantly higher MD, RD, and AD values in distal mutation. In contrast, the proximal mutation demonstrates spatially localized abnormal FA values with no significant changes in MD. Similar findings were also noted on atlas-based analysis of tract diffusion metrics.
Conclusion: Our DTI finding suggests that DMD children with proximal mutation preserved the brain microstructural integrity while DMD children with distal mutation demonstrate widespread structural WM abnormalities with the poor clinical and neuropsychological profile.
Acknowledgements
No acknowledgement found.References
1. Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010;9:77–93.
2. Pane M, Lombardo ME, Alfieri P, et al. Attention deficit hyperactivity disorder and cognitive function in Duchenne muscular dystrophy: phenotype-genotype correlation. J Pediatr 2012;161:705-709.e1.
3. D’Angelo MG, Lorusso ML, Civati F, et al. Neurocognitive profiles in Duchenne muscular dystrophy and gene mutation site. Pediatr Neurol 2011;45:292–9.
4. Banihani R, Smile S, Yoon G, et al. Cognitive and Neurobehavioral Profile in Boys With Duchenne Muscular Dystrophy. J Child Neurol 2015;30:1472–82.
5. Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol 2003;2:731–40.
6. Doorenweerd N, Mahfouz A, van Putten M, et al. Timing and localization of human dystrophin isoform expression provide insights into the cognitive phenotype of Duchenne muscular dystrophy. Sci Rep 2017;7:12575.
7. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215.
8. Taylor PJ, Betts GA, Maroulis S, et al. Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PloS One 2010;5:e8803.
9. Cotton S, Voudouris NJ, Greenwood KM. Intelligence and Duchenne muscular dystrophy: full-scale, verbal, and performance intelligence quotients. Dev Med Child Neurol 2001;43:497–501.
10. Snow WM, Anderson JE, Jakobson LS. Neuropsychological and neurobehavioral functioning in Duchenne muscular dystrophy: A review. Neurosci Biobehav Rev 2013;37:743–52.
11. Hinton VJ, Fee RJ, Goldstein EM, et al. Verbal and memory skills in males with Duchenne muscular dystrophy. Dev Med Child Neurol 2007;49:123–8.
12. Fu Y, Dong Y, Zhang C, et al. Diffusion tensor imaging study in Duchenne muscular dystrophy. Ann Transl Med 2016;4.
13. Acosta-Cabronero J, Williams GB, Pengas G, et al. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain J Neurol 2010;133:529–39.
Figures