0076
Mapping fetal brain development based on automated brain segmentation and 4D brain atlasing1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, Zhejiang, China, 2Department of Radiology, Women's Hospital,School of Medicine,Zhejiang University, Hangzhou, Zhejiang, China, 3Diagnostic Imaging and Radiology, Children's National Medical Center, Washington, DC, WA, United States
Synopsis
Fetal brain MRI has become an important tool for in-utero assessment of brain development and disorders. Here we proposed an automated pipeline with fetal brain segmentation, super-resolution reconstruction, and fetal brain atlasing to quantitatively map in-utero fetal brain development in a Chinese population. We designed a U-net CNN implemented for automatic fetal brain segmentation, which showed superior segmentation accuracy compared with conventional methods. We then generated a Chinese fetal brain atlas, using an iterative linear and nonlinear registration method. Based on the 4D spatiotemporal atlas, we characterized the three-dimensional morphological evolution of the fetal brain between 23-36 weeks of gestation.
Introduction
The field of fetal brain MRI has grown rapidly due to the development of fast image acquisition techniques and advanced computational tools1,2, although many challenges remain. Volumetric reconstruction of the fetal brain from 2D images using the super-resolution technique3-5 is commonly used, which, however, requires extraction of the fetal brain from the uterus prior to the reconstruction. Manual delineation of the brain from stacks of 2D slices in all three orientations is labor-intensive, especially for large datasets, and conventional brain extraction methods usually fail. Therefore, we proposed a modified U-net6 model-based deep learning approach to segment the fetal brain. Using this automated approach, we were able to reconstruct healthy fetal brains across gestation and generate the first version of Chinese fetal brain atlases that allowed us to depict normal in-utero fetal brain development.Methods
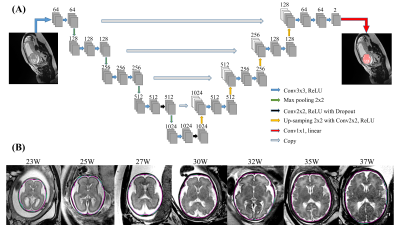
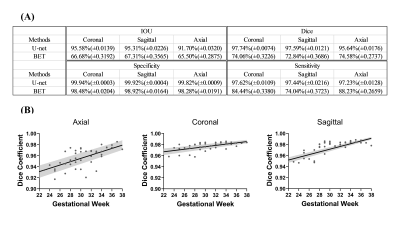
Data acquisition: In this study, 2D multi-slice T2-weighed images were retrospectively obtained from 212 fetuses between 21-40 weeks gestational age, after visual inspection for the image quality. The images were acquired on a 1.5T GE scanner (Signa Hdxt) using T2-prepared balanced SSFP with TE/TR = 2.1/4.7ms and flip angle of 55º, or single-shot FSE with TE/TR=130/2400ms, at in-plane resolution around 0.74×0.74mm (512×512 matrix), and 12-16 slices with slice-thickness of 4-5mm, in axial, coronal, and sagittal orientations.Fetal brain segmentation: Figure 1A shows the proposed network structure for fetal brain segmentation. The convolution layer was set to have a kernel size of 3×3, stride of 2, and zero padding. The input slices were augmented ten times randomly by image translation, rotation, cropping, and mirror symmetry. The manually delineated fetal brain contours were used as ground truth and the cross-entropy was calculated within the brain masks, as the cost function. The performance was evaluated by the intersection over union (IOU), Dice coefficient, sensitivity, and specification, with comparison to brain extraction tools (BET) in FSL7.
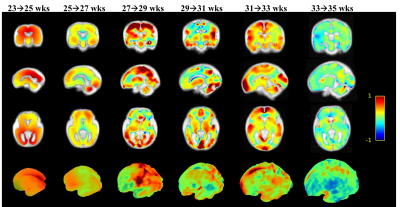
Fetal brain atlasing: 35 healthy fetal brains scanned with bSSFP sequence between gestational weeks 23-36 were used to generate the 4D atlas. 3D volumes were reconstructed from the segmented 2D slices using the super-resolution method8 (https://github.com/rousseau/fbrain). We selected five brains from every two gestational weeks to generate a population template. The pipeline of the atlas generation is shown in Figure 2. In the first iteration, one of the fetal brains was selected as the initial template (IA1) and the remaining images were registered to the reference image by affine registration and Large Deformation Diffeomorphic Metric Mapping (LDDMM) registration9, to obtain the averaged template (IA2). In the second iteration, all five brains were transformed to IA2, and the procedure was repeated 3-4 times until the template became stable.
Quantification of fetal brain development: We removed the cerebrospinal fluid for every template and then registered the adjacent templates using the LDDMM algorithm. The determinant of the log-Jacobian matrix from the transformation was used to quantify the morphological changes between adjacent gestational stages.
Results
Figure 1B shows representative segmentation results of fetal brains at different gestational ages. The red contours, which denote the automated segmentation by the U-net, mostly overlapped with the green contours that denote the manually segmented ground truth. The quantitative measures in Figure 3A demonstrated that the proposed U-net method yielded an average Dice of 0.97 across the three brain orientations. In comparison, the BET method resulted in an average Dice of 0.74. Noticeably, the segmentation accuracy showed an age-dependency (Figure 3B), likely due to the relatively small number of training data at small gestational ages.We constructed fetal brain atlases by computing the atlas templates every two weeks from 23 to 36 gestational weeks. The 4D spatiotemporal fetal brain atlas is shown in Figure 4 and the morphological changes between adjacent atlases are illustrated in Figure 5 in 2D and 3D views. Dramatic fetal brain growth was observed during early gestation, e.g., from 23 to 25 weeks, and the growth rate slowed down towards late gestation. Moreover, a posterior-to-anterior developing pattern was observed, e.g., by comparing the log-Jacobian map from 27 to 29 weeks and that from 31 to 33 weeks.
Discussion and Conclusion
In this work, we proposed a fully automatic segmentation method based on U-Net and an atlas-based method to quantitatively map fetal brain development. We constructed a 4D spatiotemporal fetal atlas from 23-36 weeks of gestation in a Chinese population, which to the best of our knowledge is the first Chinese fetal brain atlas. Although high-quality fetal brain atlases in Caucasian populations have been reported10,11 (http://crl.med.harvard.edu/research/fetal_brain_atlas/), several comparative studies showed considerable anatomical differences between races in children and adults12. It is possible the developmental differences begin in the fetal period, and therefore, it is essential to build a racial specific fetal brain atlas for related studies.There are several limitations to the current study. All of our imaging data were retrospectively collected from pure clinical data, which typically had low resolution (thick slices), and therefore, the reconstructed 3D images may not match the existing high-resolution fetal brain atlas10. The low slice resolution limited more detailed analysis, such as GM and WM segmentation, however, they were sufficient for morphological analysis as we have shown and are suitable for future analysis of clinical fetal MRI data.
Acknowledgements
This work was supported by the Natural Science Foundation of China (61801424, 81971606, and 91859201) and the Ministry of Science and Technology of the People’s Republic of China (2018YFE0114600).References
1. Studholme C. Mapping fetal brain development in utero using magnetic resonance imaging: the Big Bang of brain mapping. Annu Rev Biomed Eng. 2011; 13:345-368.
2. Jarvis DA, Griffiths PD. Current state of MRI of the fetal brain in utero. J Magn Reson Imaging. 2019; 49(3):632-646.
3. Gholipour A, Estroff JA, Warfield SK, et al. Robust super-resolution volume reconstruction from slice acquisitions: application to fetal brain MRI. IEEE T Med Imaging. 2010; 29(10):1739-1758.
4. Rousseau F, Kim K, Studholme C et al. On super-resolution for fetal brain MRI. Medical Image Computing and Computer-Assisted Intervention, Springer Berlin Heidelberg. 2010; 355-362.
5. Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA et al. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Anal. 2012; 16(8):1550-1564.
6. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional networks for biomedical image segmentation. Medical Image Computing and Computer-Assisted Intervention, Springer Cham. 2015; 234-241.
7. Jenkinson M, Pechaud M, Smith S, et al. BET2: MR-based estimation of brain, skull and scalp surfaces. Eleventh Annual Meeting of the Organization for Human Brain Mapping. 2005; 17:167.
8. Rousseau F, Oubel E, Pontabry J et al. BTK: An open-source toolkit for fetal brain MR image processing. Comput Meth Prog Bio. 2013; 109(1):65-73.
9. Beg MF, Miller MI, Trouvé A, et al. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. Int J Comput Vision. 2005; 61(2):139-157.
10. Gholipour A, Rollins CK, Velasco-Annis C, et al. A normative spatiotemporal MRI atlas of the fetal brain for automatic segmentation and analysis of early brain growth. Sci Rep. 2017; 7(1):476.
11. Khan S, Vasung L, Marami B, et al. Fetal brain growth portrayed by a spatiotemporal Diffusion Tensor MRI Atlas computed from in utero images. NeuroImage. 2018; 185:593-608.
12. Rao NP, Jeelani H, Achalia R, et al. Population differences in brain morphology: Need for population specific brain template. Psychiat Res-Neuroim. 2017; 265:1-8.
Figures