0069
Quantitative Susceptibility Mapping using a Multi-spectral ARMA Model for Assessment of Hepatic Iron Overload
Aaryani Tipirneni-Sajja1,2, Ralf Berthold Loeffler2,3, Jane Hankins4, and Claudia Maria Hillenbrand2,3
1Biomedical Engineering, University of Memphis, Memphis, TN, United States, 2Diagnostic Imaging, St. Jude Children's Research Hospital, Memphis, TN, United States, 3Research Imaging NSW, University of New South Wales, Sydney, Australia, 4Hematology, St. Jude Children's Research Hospital, Memphis, TN, United States
1Biomedical Engineering, University of Memphis, Memphis, TN, United States, 2Diagnostic Imaging, St. Jude Children's Research Hospital, Memphis, TN, United States, 3Research Imaging NSW, University of New South Wales, Sydney, Australia, 4Hematology, St. Jude Children's Research Hospital, Memphis, TN, United States
Synopsis
Hepatic iron content (HIC) assessment by R2*-MRI can be confounded by co-existing fibrosis. Instead, quantitative susceptibility mapping (QSM) techniques could be used to assess iron content without being affected by fibrosis. In this study, we demonstrated that the field maps generated from a multi-spectral auto regressive moving average (ARMA) model can be used in conjunction with QSM techniques to measure magnetic susceptibility, as a predictor for HIC.
Introduction
R2*-Magnetic resonance imaging (MRI) has emerged as a noninvasive and longitudinal monitoring technique for assessment of hepatic iron overload. A major challenge with this method, however, is that any co-existing pathologies such as fatty infiltration and fibrosis affect R2* measurements, and hence would interfere with HIC estimation. In recent years, multispectral signal modeling techniques such as autoregressive moving average (ARMA) model has demonstrated to simultaneously quantify R2* and fat fraction (FF); but these techniques are still confounded by fibrosis.1,2 Alternatively, quantitative susceptibility mapping (QSM) could be used to assess iron content without being affected by fibrosis.1 QSM techniques have been well-validated in brain applications for quantifying iron deposits.3 However, these techniques have been challenging to implement in abdomen due to problems related to breathing motion, the presence of subcutaneous/liver fat and/or severe iron overload that may all hinder the accurate estimation of field map.4 In this work, we propose to evaluate if ARMA generated field maps can be used in conjunction with QSM techniques for assessment of hepatic iron overload.Materials and Methods
Ten 1L iron phantoms were constructed from 2% agar-water mixtures and doped with various amounts of bionized nonferrites particles to obtain a wide range of R2* values. In vivo data covering the entire liver were collected from 27 patients who underwent MRI scans for clinical monitoring of hepatic iron overload. All patients were scanned on a 1.5T scanner (Magnetom Avanto, Siemens Healthineers, Malvern, PA) using a 3D multi-echo gradient echo (GRE) sequence (TR/TE/ΔTE = 16/1.41/1.6 ms, 6 echoes, flip angle = 60, FOV = 350 mm, matrix = 320 x 280, slice thickness = 3.5 mm). Multi-spectral ARMA modeling was performed on the complex GRE signal acquired in phantoms and patients to calculate R2* and FF values, and estimate field map via an iterative Stieglitz-McBride algorithm.1,2 The estimated field map was further processed using the projection-onto-dipole-field (PDF) method to remove background field and produce a local field map.5 Finally, susceptibility maps were generated from the local field maps using the morphology enabled dipole inversion algorithm.5 Mean R2* and susceptibility values were calculated by drawing circular ROIs in phantoms and were correlated to iron concentrations. In patients, 2 circular ROIs were drawn in homogeneous areas of liver and muscle tissues and the mean susceptibility values were calculated with reference to muscle. Both R2* and susceptibility values obtained using ARMA were correlated to HIC values estimated using a previously published calibration curve.6Results and Discussion
In phantoms, susceptibility values estimated using ARMA field maps showed a strong linear relationship with iron concentrations (R2 = 0.9). Representative examples of ARMA calculated R2*, susceptibility and fat fraction maps in mild (3 < HIC < 7 mg Fe/g), moderate (7 < HIC < 15 mg Fe/g), and high (HIC > 15 mg Fe/g) cases of iron overload are shown in Figure 2. The ARMA estimated FF values were <5% in all cases as our cohort predominantly consists of patients with hepatic iron overload due to chronic blood transfusions; mainly sickle cell patients with low BMI. The susceptibility maps generated using the ARMA model for field map estimation were homogenous with good anatomical depiction of vessels and tissue boundaries. The R2* and susceptibility values calculated using ARMA model demonstrated a high and a moderate linear correlation with the HIC values, respectively (Fig. 3). Although the correlation between HIC and susceptibility values is less than R2*, our results show the possibility of using ARMA field maps in conjunction with QSM techniques to generate susceptibility maps for assessment of hepatic iron overload. Future work will focus on optimizing the acquisition parameters to improve the estimation of ARMA field map and hence susceptibility maps and to finally test these methods in iron overloaded patients with co-existing fibrosis and steatosis.Conclusion
Our preliminary results show that the ARMA model can be used as a comprehensive technique to estimate susceptibility maps in addition to R2* and FF maps. This method should be further tested in patients with co-existing iron overload, fibrosis and/or fatty liver in order to evaluate the potential value of QSM in assessing iron overload with co-existing pathologies.Acknowledgements
No acknowledgement found.References
1) Taylor BA JMRI 2012; 2) Tipirneni-Sajja A JMRI 2019; 3) Liu T Magn Reson Med 2011; 4) Sharma SD MRM 2015; 5) Wang Y MRM 2015; 6) Hankins JS Blood 2009Figures
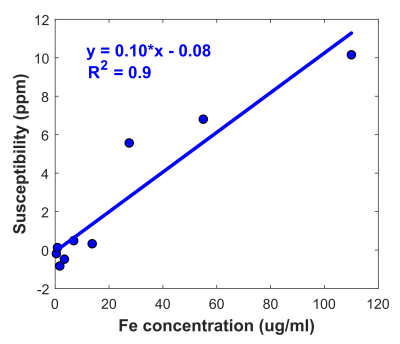
Figure 1. Linear regression analysis
between mean QSM values obtained using ARMA field maps and iron concentrations
in phantoms.
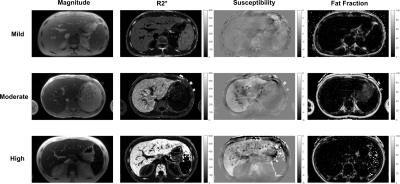
Figure 2. Magnitude images, R2*, QSM and fat fraction maps obtained
using ARMA model in mild, moderate and high iron overload cases.
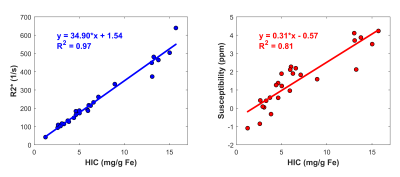
Figure 3. Linear regression analysis
between R2* values (a), and QSM values (b) generated with ARMA and HIC values in
patients with iron overload.