0068
Virtual bronchoscopy of neonatal dynamic airway collapse with ultrashort echo-time MRI1Center for Pulmonary Imaging, Cincinnati Children's Hospital, CINCINNATI, OH, United States, 2Pulmonary Medicine, Cincinnati Children's Hospital, CINCINNATI, OH, United States, 3Upper Airway Center, Cincinnati Children's Hospital, CINCINNATI, OH, United States, 4Pediatrics, University of Cincinnati, CINCINNATI, OH, United States, 5Radiology, Cincinnati Children's Hospital, CINCINNATI, OH, United States, 6Pediatrics, University of Cincinnati, Cincinnati, OH, United States, 7Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 8Radiology, University of Wisconsin - Madison, Madison, WI, United States, 9Neonatology and Pulmonary Biology, Cincinnati Children's Hospital, CINCINNATI, OH, United States
Synopsis
Central airway abnormalities in neonates, e.g. dynamic collapse and stenosis, are serious complications often associated with preterm birth and congenital abnormalities, but have not been extensively studied. These conditions are most often assessed through clinical bronchoscopy, which can be unreliable and poses increased risks to patients. Here, we demonstrate novel visualization of static and dynamic neonatal airway abnormalities on virtual bronchoscopy from high-resolution, retrospectively respiratory-gated ultrashort echo-time MRI, which exhibits good agreement with clinical bronchoscopy. This virtual technique allows for assessment of neonatal airway abnormalities that is readily interpretable to clinicians familiar with clinical bronchoscopy.
INTRODUCTION
Dynamic collapse of the central airway (e.g. laryngomalacia [LM], tracheomalacia [TM], or bronchomalacia [BM]) is a serious and common complication occurring in neonates with bronchopulmonary dysplasia (BPD, lung disease of prematurity) and congenital tracheoesophageal defects (TED).1–6 However, the underlying pathologies, impact on clinical outcomes, and response to therapies are not well understood.5–7 Clinical bronchoscopy (CB) is the current standard for assessing neonatal airway conditions8 but has several limitations,9 in particular that it is invasive and typically requires sedation/anesthesia. As such, the procedure poses increased risks to small, vulnerable infants and limits implementation in broad infant populations. Furthermore, invasive bronchoscopy under sedation affects the natural airway state and may alter the structure, dynamics, and airflow under investigation.Imaging-based virtual bronchoscopy (VB) has been proposed as an alternative diagnostic tool but thus far has had limited clinical relevance.10 To date, most VB has been performed using computed tomography (CT) images. However, this modality faces various obstacles in infants: airway collapse can be visualized by CT but the temporal resolution is limited by gantry rotation; CT is relatively uncommon in neonates due to concerns over ionizing radiation, even with low-dose protocols; and furthermore, CT typically requires sedation/anesthesia, obscuring natural airway dynamics.
Our group has previously developed an MRI-based technique for regional quantification of neonatal airway dynamics using respiratory-gated, 3D radial ultrashort echo time (UTE) MRI,11 which demonstrates good correlation between tracheal collapse quantification and both bronchoscopic severity diagnosis12 and need for tracheostomy.13 While quantitative analysis yields more objective assessment of airway collapse than current techniques allow, it is less familiar to clinicians who typically assess neonatal airway abnormalities via bronchoscopy.
In this study, we demonstrate novel visualization of dynamic neonatal airway abnormalities on VB via respiratory-gated, 3D UTE MRI, which shows good agreement with CB. This virtual technique allows for “visual” assessment of neonatal airway dynamics similar to that of CB and thus is readily interpretable to clinicians. Importantly, this technique is implemented during quiet breathing conditions, without requiring invasive procedures, sedation/anesthesia, or ionizing radiation.
METHODS
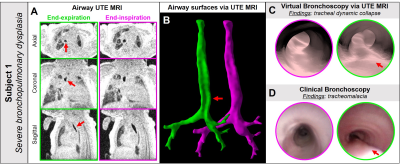
Six neonatal in-patients were recruited (demographics summarized in Figure-1). Airway MRI was acquired during tidal breathing using a 3D radial 1H UTE sequence14,15 and quadrature body coil on a neonatal-sized 1.5T scanner,16,17 with no sedation ordered for imaging. Typical UTE acquisition parameters were: TR/TE=5/0.2ms; FA=5º; FOV=18cm; number of radial projections=200,000; isotropic resolution=0.70mm; and scan time=16min. Superior-inferior image coverage included the oropharynx through to the lung base.Using the motion-modulated k0 time-course for retrospective respiratory gating,18 images were reconstructed into end-expiration and end-inspiration frames with 20% hard-gating acceptance windows (Figure-2). Airway anatomy was segmented semi-automatically with seed-growing on gated UTE images via ITK-SNAP (3.6.0, Penn Image Computing and Science Laboratory, USA).19 Surfaces were smoothed with Taubin smoothing (λ=0.6, µ=-0.6, 10 steps), with VB visualized in ParaView 5.6.0 (Figure-2). Imaging-based airway collapse was defined as ≥30% change in airway luminal cross-sectional area between end-expiration and end-inspiration. For subjects who also underwent CB, virtual and clinical findings were qualitatively compared.
RESULTS
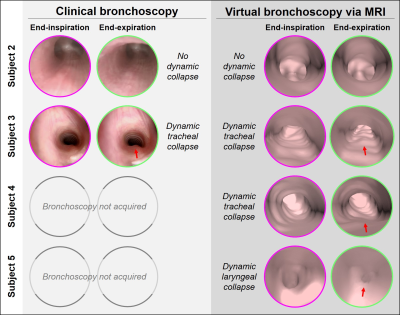
Dynamic collapse in the trachea (TM) was identified on VB in 4/6 subjects, dynamic collapse in the larynx (LM) was identified in 1/6 subject, and an absence of dynamic airway collapse was identified in 1/6 subject (Figure-1).4/6 subjects (Subjects 1-3 and 6) also underwent CB, with findings of dynamic collapse from CB and VB in qualitative agreement (Figure-3). Importantly, 2/6 MRI subjects (Subjects 4 and 5) did not undergo CB as airway issues were not clinically suspected, yet dynamic collapse was apparent on VB (Figure-3). In Subject 6, significant static tracheal narrowing, presence and location of tracheoesophageal fistula defect, and dynamic tracheal collapse were identified on both CB and VB (Figure-4).
DISCUSSION AND CONCLUSIONS
We have presented a novel MRI technique for VB providing regional, visual assessment of neonatal dynamic airway abnormalities with bronchoscopic views that are familiar to clinicians, without requiring invasive, ionizing, sedated, or breath-holding procedures. MRI-based VB qualitatively compares well to CB. While rigorous validation is required to establish accuracy, this method overcomes the current barriers associated with existing diagnostic standards, in that scans can be performed during natural breathing in neonatal patients and thus airway dynamics are unaffected.Previous work has found that CT-based VB of airway dynamics in young pediatrics (median age = 2.5 years) has moderate sensitivity but high specificity and positive predictive value;20 with similar spatial resolution, we hypothesize that MRI-based VB of neonatal airways would have similar diagnostic accuracy. While MRI-based VB may not replace invasive bronchoscopy, it poses no additional risks to patients and may have a role as an initial minimal-risk assessment to determine whether a neonatal patient would benefit from undergoing CB. Furthermore, this VB technique can be used in conjunction with previously published MRI methods for quantification of neonatal airway collapse,11 which is not possible by CB.
In the future, we envision that MRI-based VB may be used in serial assessment of therapeutic interventions for airway collapse (e.g. bethanechol). Ultimately, this approach has the potential to advance understanding and treatment of airway morbidities in this vulnerable neonatal population, with minimal additional risk.
Acknowledgements
The authors would like to acknowledge support from the Cincinnati Children’s Research Foundation, The Hartwell Foundation at University of Wisconsin – Madison, and NIH T32 HL007752, R01 HL146689, P01 HD093363.References
1. Downing GJ, Kilbride HW. Evaluation of airway complications in high-risk preterm infants: application of flexible fiberoptic airway endoscopy. Pediatrics. 1995;95(4):567-572. http://www.ncbi.nlm.nih.gov/pubmed/7700760.
2. Miller RW, Woo P, Kellman RK, Slagle TS. Tracheobronchial abnormalities in infants with bronchopulmonary dysplasia. J Pediatr. 1987;111(5):779-782. doi:10.1016/S0022-3476(87)80267-6
3. Cohn RC, Kercsmar C, Dearborn D. Safety and Efficacy of Flexible Endoscopy in Children With Bronchopulmonary Dysplasia. Am J Dis Child. 1988;142(11):1225-1228. doi:10.1001/archpedi.1988.02150110103030
4. McCubbin M, Frey EE, Wagener JS, Tribby R, Smith WL. Large airway collapse in bronchopulmonary dysplasia. J Pediatr. 1989;114(2):304-307. doi:10.1016/S0022-3476(89)80802-9
5. Hysinger EB, Friedman NL, Padula MA, et al. Tracheobronchomalacia is associated with increased morbidity in bronchopulmonary dysplasia. Ann Am Thorac Soc. 2017;14(9):1428-1435. doi:10.1513/AnnalsATS.201702-178OC
6. Hysinger EB, Panitch HB. Paediatric tracheomalacia. Paediatr Respir Rev. 2016;17:9-15. doi:10.1016/j.prrv.2015.03.002
7. Baxter JD, Dunbar JS. Tracheomalacia. Ann Otol Rhinol Laryngol. 1963;(72):1013:23. http://journals.sagepub.com/doi/pdf/10.1177/000348946307200415. Accessed April 27, 2018.
8. Hysinger E, Friedman N, Jensen E, Zhang H, Piccione J. Bronchoscopy in neonates with severe bronchopulmonary dysplasia in the NICU. J Perinatol. 2019;39(2):263-268. doi:10.1038/s41372-018-0280-y
9. Burg G, Hysinger E, Hossain M, Wood R. Evaluation of Agreement on Presence and Severity of Tracheobronchomalacia in Pediatric Patients by Dynamic Flexible Bronchoscopy. In: Proceedings of the American Thoracic Society. ; 2018:197:A3660.
10. Miyoshi S, Isobe K, Shimizu H, et al. The Utility of Virtual Bronchoscopy Using a Computed Tomography Workstation for Conducting Conventional Bronchoscopy: A Retrospective Analysis of Clinical Practice. Respiration. 2019;97(1):52-59. doi:10.1159/000492074
11. Bates A, Higano N, Hysinger E, et al. Quantitative Assessment of Regional Dynamic Airway Collapse in Neonates via Retrospectively Respiratory-Gated 1 H Ultrashort Echo Time MRI. J Magn Reson Imaging. 2018. doi:doi: 10.1002/jmri.26296. [Epub ahead of print]
12. Hysinger E, Bates A, Higano N, et al. Quantitative Assessment of Tracheomalacia Using Ultra-Short Echo Time MRI. In: Proceedings of the American Thoracic Society. San Diego, CA; 2018:197:A4466.
13. Higano NS, Bates AJ, Hysinger EB, et al. Dynamic Tracheal Collapse and Correlation to Later Tracheostomy in Neonates with Bronchopulmonary Dysplasia Via Quantitative Ultrashort Echo-Time MRI. In: C16. CLINICAL STUDIES IN BRONCHOPULMONARY DYSPLASIA. ; :A4264-A4264. doi:10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A4264
14. Hahn AD, Higano NS, Walkup LL, et al. Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging. 2017;45(2):463-471. doi:10.1002/jmri.25394
15. Higano NS, Fleck RJ, Spielberg DR, et al. Quantification of neonatal lung parenchymal density via ultrashort echo time MRI with comparison to CT. J Magn Reson Imaging. 2017;46(4):992-1000. doi:10.1002/jmri.25643
16. Tkach JA, Hillman NH, Jobe AH, et al. An MRI system for imaging neonates in the NICU: initial feasibility study. Pediatr Radiol. 2012;42(11):1347-1356. doi:10.1007/s00247-012-2444-9
17. Tkach JA, Merhar SL, Kline-Fath BM, et al. MRI in the neonatal ICU: Initial experience using a small-footprint 1.5-T system. Am J Roentgenol. 2014;202(1). doi:10.2214/AJR.13.10613
18. Higano NS, Hahn AD, Tkach JA, et al. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med. 2017;77(3):1284-1295. doi:10.1002/mrm.26212
19. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116-1128. doi:10.1016/j.neuroimage.2006.01.015
20. Su SC, Masters IB, Buntain H, et al. A comparison of virtual bronchoscopy versus flexible bronchoscopy in the diagnosis of tracheobronchomalacia in children. Pediatr Pulmonol. 2017;52(4):480-486. doi:10.1002/ppul.23606
Figures