0066
Reduced arterial compliance-mediated neural-vascular uncoupling underlies cognitive impairment in multiple sclerosis1The University of Texas at Dallas, Dallas, TX, United States, 2Johns Hopkins University, Baltimore, MD, United States, 3University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
Cognitive impairment occurs in ~70% of multiple sclerosis patients (MSP). The neural mechanism of this slowing is unknown. Vascular compliance reductions along the cerebrovascular tree would result in suboptimal vasodilation upon neural stimulation (i.e., neural-vascular uncoupling) and thus cognitive slowing. We tested arterial and venous cerebrovascular reactivity (CVR) along the cerebrovascular tree in nested cerebral cortical layers. Arterial CVR reduced exponentially along the cortical layers in controls and cognitively-normal MSP, but not in slower MSP. The exponential decay-constant was associated with individual subjects’ reaction-time. Such associations implicate neural-vascular uncoupling as a mechanism of cognitive slowing in MS.
Introduction
Cognitive impairment occurs in ~70% of multiple sclerosis (MS) patient.1,2 The pathophysiology of this impairment is unknown. Neural-vascular coupling, acute localized blood flow increases following neural activity is essential for efficient cognition.3 Loss of vascular compliance along the cerebrovascular tree (i.e., vascular network extending from superficial pial arteries to deep penetrating arteries) would result in suboptimal vasodilation, providing insufficient nutrients and oxygen to active neurons.4 This process is called neural-vascular uncoupling and we hypothesized this uncoupling would result in cognitive impairment.Methods
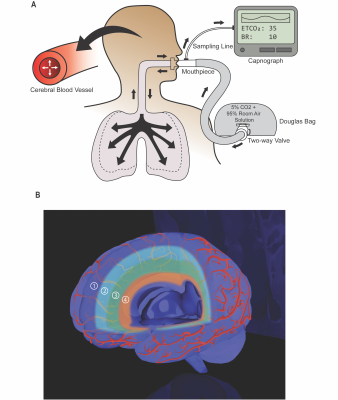
We conducted a cross-sectional study investigating 17 healthy controls (HCs), 20 cognitively normal MS and 13 cognitively impaired MS patients. Participants were scanned on a Philips 3T MRI scanner using a dual-echo functional MRI sequence and acquired blood-oxygen-level-dependent signal (BOLD) and cerebral blood flow (CBF) near-simultaneously. Participants periodically inhaled room-air and hypercapnic gas-mixture (5% CO2 and 95% room air) while undergoing the scan.5 Arterial and venous cerebrovascular reactivity (CVR) was calculated from both hypercapnia-evoked cerebral blood flow (arterial) and blood-oxygen-level-dependent signal (venous) increases per-unit increase in end-tidal CO2.6 CVR was studied in sequential layers from the exterior to interior of cerebral cortex (Fig 1). This method allowed us to assess compliance of arteries and veins along the cerebrovascular tree. The compliance of penetrating arteries and parenchymal arterioles, and veins, along the cortical layers from the exterior to interior was measured by the arterial and venous compliance indices respectively. Inspecting HCs’ group mean arterial CVR and venous CVR in layers 1-4 (Fig 2A,2B), it is apparent that the CVR exponentially decreased from layers 1 to 4. Therefore, ACI was calculated as the decay-constant of the exponential function in arterial CVR moving inward from the outermost cortical layer (i.e., Δarterial CVR/Δlayer; Eq. 1). Venous compliance index (VCI) was calculated as the decay-constant of the exponential function in venous CVR moving inward from the outermost cortical layer (i.e., Δvenous CVR/Δlayer; Eq. 2). $$ Arterial\ CVR\ =\ e^{ACI*layer}\ (1)$$ $$Venous\ CVR\ =\ e^{VCI*layer}\ (2)$$Results
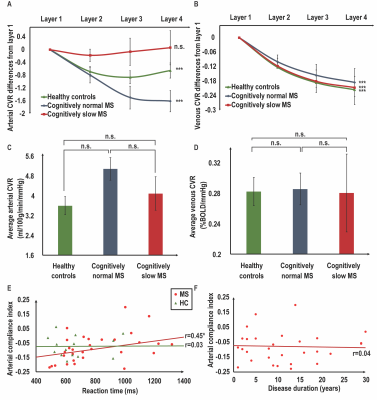
1. Altered arterial compliance along the cerebrovascular tree for slow MS patients.We sought to assess the reactivity of penetrating arteries and parenchymal arterioles along the cerebrovascular tree by testing for changes in arterial CVR sequentially moving from layer 1 to 4 between the three groups. We tested the hypothesis of group-differences in arterial CVR changes using a 4 (Layer) by 3 (Group) mixed ANOVA. There was a main effect of Layer on arterial CVR, F(1.349, 55.313)=11.359, p=0.0005. The Group main effect was not significant (p=0.0775). There was a significant interaction between Layer and Group on arterial CVR, F(2.698, 55.313)=4.605, p=0.0077. Arterial CVR decreased sequentially from layers 1 to 4 in HCs, F(1.542, 20.048)=10.638, p=0.0014 and cognitively normal MS patients, F(1.287, 24.455)=18.289, p=0.0005, (Fig. 2A). In cognitively slow MS patients, there were no changes in arterial CVR sequentially from layers 1 to 4, p=0.7244 (Fig 2A). As a result of CO2-evoked arterial vasodilation along the cerebrovascular tree, local oxygenated-hemoglobin in the venous system increases. We sought to test the compliance of the venous system to accommodate increases in local oxygenated hemoglobin by testing for changes in venous CVR sequentially from layers 1 to 4 between the three groups. The main effect of Layer showed significant differences in venous CVR at different layers, F(1.032,42.293)=69.436, p=0.0005; Fig. 2B. The main effect of Group was not significant (p=0.9911). The interaction between Layer and Group on venous CVR was not significant (p=0.8193). There were no differences in arterial and venous CVR averaged across layers between the three groups (Fig. 2C, 2D).
2. ACI explains more variability in reaction-time (RT) after controlling for MS-disease process variables.
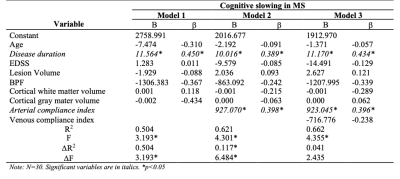
Hierarchical regression was performed to test the hypothesis that ACI predicted reaction time (RT; a measure of processing speed) in MS patients after accounting for MS-disease process variables (Table 3). The model of Age, Disease Duration, Expanded-Disability-Status-Scale, Lesion Volume, Brain Parenchymal Fraction, Grey Matter Volume, and White Matter Volume (Fig. 3,Model 1) to predict RT was statistically significant, R2=0.504, F(7, 22)=3.193, p=0.0172, adj. R2=0.346. The addition ACI (Fig. 3,Model 2) to the RT prediction in MS patients led to a statistically significant increase in ΔR2of 0.117, F(8, 21)=4.301, p=0.0034. This result suggests that ACI explains 11.7% of simple RT variability in MS patients after accounting for MS-disease process variables. The addition of VCI (Fig. 3,Model 3) to RT prediction did not lead to significant increase in R2 (p=0.1343).
Discussion
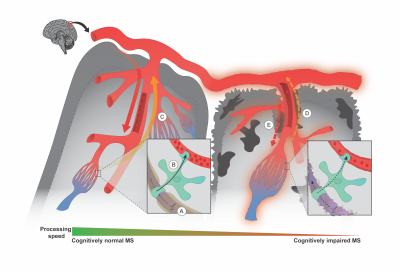
The present results are consistent with emerging evidence that the neural-glial-vascular system becomes dysfunctional in MS.6,7,8,9 This study found arterial CVR alterations along the cerebrovascular tree in MS patients whose cognitive performance was impaired compared to patients with normal performance and HCs. This alterations along the cerebrovascular tree would result in abnormal CBF response to the neurometabolic demand and thus, neural-vascular uncoupling.9 Such uncoupling would result in altered neural function and cognitive slowing (Fig. 4).Conclusion
ACI is a novel biomarker reflecting the degree of injury along the cerebrovascular tree. This index might provide a platform for MS disease surveillance, development of targeted cognitive therapies, and a method for distinguishing between MS patients who are prone to develop cognitive impairment and those who are not.Acknowledgements
We thank all the patients and volunteers who participated in this research efforts. We thank our lab research interns and coordinators for their help in recruiting patients and volunteering for the study.References
1. Genova HM, Hillary FG, Wylie G, et al. Examination of processing speed deficits in multiple sclerosis using functional magnetic resonance imaging. Epub ahead of print 2009. DOI: 10.1017/S1355617709090535.
2. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol2008; 7: 1139–1151.
3. Abdelkarim, D., Zhao, Y., Turner, M.P., Sivakolundu, D.K., Lu, H., Rypma, B., 2019. A neural-vascular complex of age-related changes in the human brain: Anatomy, physiology, and implications for neurocognitive aging. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2019.09.005
4. Chen BR, Kozberg MG, Bouchard MB, et al. A Critical Role for the Vascular Endothelium in Functional Neurovascular Coupling in the Brain. J Am Heart Assoc2014; 3: e000787.
5. Lu, H., Liu, P., Yezhuvath, U., Cheng, Y., Marshall, O., Ge, Y., 2014. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J. Vis. Exp. https://doi.org/10.3791/52306
6. Marshall O, Lu H, Brisset J-C, et al. Impaired Cerebrovascular Reactivity in Multiple Sclerosis. JAMA Neurol2014; 71: 1275.
7. Hubbard NA, Turner M, Hutchison JL, et al. Multiple sclerosis-related white matter microstructural change alters the BOLD hemodynamic response. J Cereb Blood Flow Metab2016; 36: 1872–1884.
8. Jakimovski D, Benedict RH, Marr K, et al. Lower total cerebral arterial flow contributes to cognitive performance in multiple sclerosis patients. Mult Scler J2019;
9. Turner MP, Hubbard NA, Sivakolundu DK, et al. Preserved canonicality of the BOLD hemodynamic response reflects healthy cognition: Insights into the healthy brain through the window of Multiple Sclerosis. Neuroimage. Epub ahead of print 2018. DOI: 10.1016/j.neuroimage.2017.12.081.
Figures