0064
Regional oxygen extract fraction mapping (rOEF) of multiple sclerosis brains1Biomedical Engineering, Cornell University, New York, NY, United States, 2Radiology, Weill Cornell Medical College, New York, NY, United States, 3Neurology, Weill Cornell Medical College, New York, NY, United States
Synopsis
Impaired energy metabolism is a major contributor to the ongoing inflammation and neurodegeneration in multiple sclerosis (MS) brains, particularly MS lesions. Cerebral regional oxygen extraction fraction mapping (rOEF) obtained from challenge-free multiecho gradient echo data demonstrates that lesions identified on quantitative susceptibility mapping (QSM) without rim (QSM rim-) have heterogenous OEF that is higher than that in other type of lesions. rOEF may offer insight into MS lesion remylination viability.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating neurologic disease and the disease progression involves neurodegeneration. One contributing tissue injury pathway is impaired energy metabolism with damaged mitochondrial ATP production (1). MRI as the method of choice MS diagnosis and follow-up (2) is being developed to image iron accumulation in proinflammatorily activated microglia/macrophages as a measure of neuroinflammation behind the sealed blood-brain-barrier using quantitative susceptibility mapping (QSM)(3,4), and to measure oxygen extraction fraction (OEF) using deoxyheme sensitive MRI (5,6). However, current OEF measurements have been limited to global and cortical venous territories, and a voxel-wise regional OEF mapping (rOEF) is critical to allow investigating tissue damage in each MS lesion individually. In this study, a recently developed QSM+qBOLD method for rOEF (7) is used to study MS lesions.Materials and Methods
rOEFPhase and magnitude of 3D multiple echo gradient echo (mGRE) signal were modeled using QSM and qBOLD, respectively (7,8). $$Y^{*},v^{*},R_{2}^{*},S_{0}^*,\chi_{nb}^{*}=\begin{array}{c}argmin\\Y,v,R_{2},S_0,\chi_{nb}\end{array}\left\{ \begin{array}{c}\begin{array}{c}w||F_{QSM}\left(Y,v,\chi_{nb}\right)-\chi||_{2}^{2}\\+||S(t)-S_{qBOLD}\left(S_{0,},Y,v,R_{2,}\chi_{nb},t\right)||_{2}^{2}+\lambda\left(\overline{OEF(Y)}-OEF_{wb}\right)^{2}\end{array}\end{array}\right\} $$ where $$$w$$$ is a weighting term. Here, the susceptibility of a voxel is the sum of susceptibilities of the blood (determined by its oxygenation) and the non-blood tissue: $$F_{QSM}(Y,v,\chi_{nb})=\left[\frac{\chi_{ba}}{\alpha}+\psi_{Hb}\cdot\Delta\chi_{Hb}\cdot\left(-Y+\frac{1-\left(1-\alpha\right)\cdot Y_{a}}{\alpha}\right)\right]\cdot v + \left(1-\frac{v}{\alpha}\right)\cdot \chi_{nb}$$ with $$$\chi_{ba}$$$ = -108.3 ppb the fully oxygenated blood susceptibility (9), $$$\alpha$$$ =0.77 the ratio between the venous and total blood volume $$$v/CBV$$$ (10), $$$\psi_{Hb}$$$ the hemoglobin volume fraction calculated from measured Hct (11-14), $$$\Delta \chi_{Hb}$$$ the susceptibility difference between deoxy- and oxyhemoglobin (15,16), venous blood volume ($$$v$$$), and non-blood tissue susceptibility ($$$\chi_{nb}$$$). The qBOLD term models the effect of blood oxygenation on the magnitude: $$S_{qBOLD}\left(t\right)=S_0\cdot e^{-R_2\cdot t}\cdot F_{BOLD}\left(Y,v,\chi_{nb},t\right)\cdot G(t)$$ with $$$G(t)$$$ a macroscopic field effect (8), $$$F_{BOLD}\left(Y,v,\chi_{nb},t\right)=exp\left(-v\cdot f_{s}\left(\delta\omega\cdot t\right)\right)$$$ (17) where $$$f_s$$$ is the signal decay by the blood vessel network (18), and $$$\delta \omega$$$ is the characteristic frequency due to the susceptibility difference between deoxygenated blood and the surrounding tissue (8): $$\delta \omega\left(Y,\chi_{nb}\right)=\frac{1}{3}\cdot \gamma \cdot B_{0}\cdot \left[Hct\cdot \Delta \chi_{0}\cdot \left(1-Y\right) + \chi_{ba}-\chi_{nb}\right]$$ with $$$\gamma$$$=267.513MHz/T the gyromagnetic ratio, $$$B_0$$$ the main magnetic field, $$$\Delta \chi_{0}=4\pi \times 0.27 ppm$$$ the susceptibility difference between fully oxygenated and fully deoxygenated red blood cells (19). The third term in the right side of Eq.1 is the physiological constraint that the whole brain average,$$$\overline{OEF(Y)}$$$, should be similar to $$$OEF_{wb}$$$, the OEF value estimated from the straight sinus (7). We used cluster analysis of time evolution to improve the robustness against noise (7).
MS patient study
12 MS patients (39 ± 7 years) who underwent 3T MRI were selected. Imaging sequences included T1-weighted (T1w), Gadolinium enhanced T1-weighted (T1W+Gd), T2-weighted (T2w), and multi-echo-gradient-echo (mGRE) (FOV=24cm, acquisition matrix size=320-416×205-320, TE1/ΔTE/= 4.5-6.7/4.1-4.8, last TE=47.7 ms, TR= 49-58 ms, slice thickness=3 mm). QSM and rOEF maps were obtained using MEDI+0 and QQ-CAT, respectively (7,20). Lesions were identified on T2w, and classified into three types relative to surrounding normal-appearing white matter (NAWM): QSM isointense (QSM-, n=46), QSM hyperintense with rim (QSM rim+, n=32) and QSM hyperintense without rim (QSM rim-, n=101). For statistical analysis (Kruskal-Wallis test) among the three lesion types, the average OEF of each lesion was referenced to the average of its corresponding NAWM. Additionally, global OEF was compared with 11 healthy controls (34 ± 12 years) using an unpaired t-test.
Results
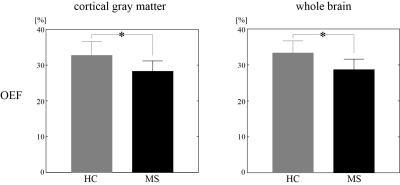
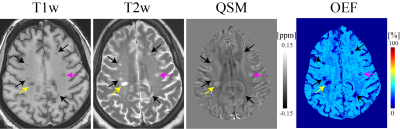
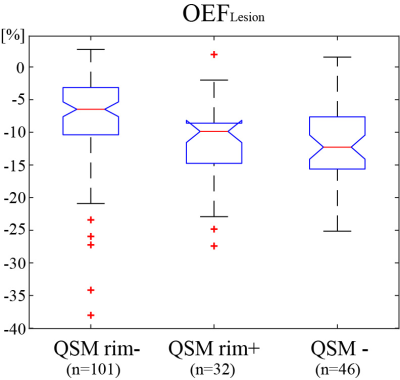
Global and cortical gray matter OEF of MS patients was significantly lower than healthy controls (33.3 ± 3.3 vs. 28.7 ± 2.9 %, p<0.01, and 32.7 ± 4.0 vs. 28.3 ± 2.9 %, p<0.01 respectively) (Fig. 1). In a MS patient with all three lesion types, QSM rim- shows heterogeneous OEF (Fig. 2). Significant OEF differences were observed among the three lesion types (p<0.01, Kruskal-Wallis test): relative to NAWM, QSM rim+, QSM rim-, QSM- showed -8 ± 7, -11 ± 6, -12 ± 6 % OEF (Fig. 3).Discussion
This is the first report on MS lesion oxygen extraction fraction, to the best of our knowledge. In the three MS white matter lesion types characterized by QSM in this study, QSM- represents old chronic lesion with little metabolism, and QSM rim+ represents lesions with substantial tissue damage (21). They present lesser energy metabolism compared to QSM rim- type (Fig.3), which is consistent with tissue damage measured on myelin water fraction mapping (22) and neuroinflammation measured on translocator protein PET (23). The heterogeneous OEF of QSM rim- lesions (Fig. 2) may reflects remyelination possibilities in these lesions.Lower global OEF in MS brains compared to healthy controls as observed here agrees well with the previously reported reduced OEF (5,6,24). The cortical OEF value here (Fig.1) is quite similar to that in a previous 7T study using vein susceptibility modeling (6), but the global OEF is slightly lower than that in a study using vein T2 modeling (5). This discrepancy may be explained by the complexity in estimating oxygenation from T2 model, particularly T2 dependence on red blood cell shape (25).
Conclusion
This study shows a voxel-wise regional OEF map in the MS. In addition to widely used T1w, T2w, and QSM, the OEF map may provide information regarding tissue viability in various MS lesions.Acknowledgements
No acknowledgement found.References
1. Aboul-Enein F, Lassmann H. Mitochondrial damage and histotoxic hypoxia: a pathway of tissue injury in inflammatory brain disease? Acta Neuropathol 2005;109(1):49-55.
2. Ge Y. Multiple sclerosis: the role of MR imaging. AJNR American journal of neuroradiology 2006;27(6):1165-1176.
3. Zhang Y, Gauthier SA, Gupta A, Comunale J, Chia-Yi Chiang G, Zhou D, Chen W, Giambrone AE, Zhu W, Wang Y. Longitudinal change in magnetic susceptibility of new enhanced multiple sclerosis (MS) lesions measured on serial quantitative susceptibility mapping (QSM). Journal of magnetic resonance imaging : JMRI 2016;44(2):426-432.
4. Zhang S, Nguyen TD, Hurtado Rúa SM, Kaunzner UW, Pandya S, Kovanlikaya I, Spincemaille P, Wang Y, Gauthier SA. Quantitative Susceptibility Mapping of Time-Dependent Susceptibility Changes in Multiple Sclerosis Lesions. American Journal of Neuroradiology 2019;40(6):987-993.
5. Ge Y, Zhang Z, Lu H, Tang L, Jaggi H, Herbert J, Babb JS, Rusinek H, Grossman RI. Characterizing brain oxygen metabolism in patients with multiple sclerosis with T2-relaxation-under-spin-tagging MRI. J Cereb Blood Flow Metab 2012;32(3):403-412.
6. Fan AP, Govindarajan ST, Kinkel RP, Madigan NK, Nielsen AS, Benner T, Tinelli E, Rosen BR, Adalsteinsson E, Mainero C. Quantitative Oxygen Extraction Fraction from 7-Tesla MRI Phase: Reproducibility and Application in Multiple Sclerosis. Journal of Cerebral Blood Flow & Metabolism 2015;35(1):131-139.
7. Cho J, Zhang S, Kee Y, Spincemaille P, Nguyen TD, Hubertus S, Gupta A, Wang Y. Cluster analysis of time evolution (CAT) for quantitative susceptibility mapping (QSM) and quantitative blood oxygen level-dependent magnitude (qBOLD)-based oxygen extraction fraction (OEF) and cerebral metabolic rate of oxygen (CMRO2) mapping. Magnetic Resonance in Medicine;0(0).
8. Cho J, Kee Y, Spincemaille P, Nguyen TD, Zhang J, Gupta A, Zhang S, Wang Y. Cerebral metabolic rate of oxygen (CMRO2) mapping by combining quantitative susceptibility mapping (QSM) and quantitative blood oxygenation level-dependent imaging (qBOLD). Magnetic resonance in medicine 2018;80(4):1595-1604.
9. Zhang J, Zhou D, Nguyen TD, Spincemaille P, Gupta A, Wang Y. Cerebral metabolic rate of oxygen (CMRO2) mapping with hyperventilation challenge using quantitative susceptibility mapping (QSM). Magnetic resonance in medicine 2017;77(5):1762-1773.
10. Hongyu A, Weili L. Cerebral venous and arterial blood volumes can be estimated separately in humans using magnetic resonance imaging. Magnetic resonance in medicine 2002;48(4):583-588.
11. Zhang J, Cho J, Zhou D, Nguyen TD, Spincemaille P, Gupta A, Wang Y. Quantitative susceptibility mapping-based cerebral metabolic rate of oxygen mapping with minimum local variance. Magn Reson Med 2017.
12. Sakai F, Nakazawa K, Tazaki Y, Ishii K, Hino H, Igarashi H, Kanda T. Regional cerebral blood volume and hematocrit measured in normal human volunteers by single-photon emission computed tomography. J Cereb Blood Flow Metab 1985;5(2):207-213.
13. Savicki JP, Lang G, Ikeda-Saito M. Magnetic susceptibility of oxy- and carbonmonoxyhemoglobins. Proceedings of the National Academy of Sciences 1984;81(17):5417-5419.
14. Hoffman R. Hematology: Basic Principles and Practice: Churchill Livingstone; 2005.
15. Zhang J, Liu T, Gupta A, Spincemaille P, Nguyen TD, Wang Y. Quantitative mapping of cerebral metabolic rate of oxygen (CMRO2) using quantitative susceptibility mapping (QSM). Magnetic Resonance in Medicine 2015;74(4):945-952.
16. Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJ. Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T(1), T(2), T*(2), and non-Lorentzian signal behavior. Magn Reson Med 2001;45(4):533-542.
17. Yablonskiy DA, Sukstanskii AL, He X. BOLD-based Techniques for Quantifying Brain Hemodynamic and Metabolic Properties – Theoretical Models and Experimental Approaches. NMR Biomed 2013;26(8):963-986.
18. Ulrich X, Yablonskiy DA. Separation of cellular and BOLD contributions to T2* signal relaxation. Magn Reson Med 2016;75(2):606-615.
19. Sukstanskii AL, Yablonskiy DA. Theory of FID NMR signal dephasing induced by mesoscopic magnetic field inhomogeneities in biological systems. Journal of magnetic resonance (San Diego, Calif : 1997) 2001;151(1):107-117.
20. Liu ZA-Ohoo, Spincemaille P, Yao Y, Zhang Y, Wang Y. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. (1522-2594 (Electronic)).
21. Chen W, Gauthier SA, Gupta A, Comunale J, Liu T, Wang S, Pei M, Pitt D, Wang Y. Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology 2014;271(1):183-192.
22. Yao Y, Nguyen TD, Pandya S, Zhang Y, Hurtado Rua S, Kovanlikaya I, Kuceyeski A, Liu Z, Wang Y, Gauthier SA. Combining Quantitative Susceptibility Mapping with Automatic Zero Reference (QSM0) and Myelin Water Fraction Imaging to Quantify Iron-Related Myelin Damage in Chronic Active MS Lesions. AJNR American journal of neuroradiology 2018;39(2):303-310.
23. Kaunzner UW, Kang Y, Zhang S, Morris E, Yao Y, Pandya S, Hurtado Rua SM, Park C, Gillen KM, Nguyen TD, Wang Y, Pitt D, Gauthier SA. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain 2019;142(1):133-145.
24. Yang R, Dunn JF. Reduced cortical microvascular oxygenation in multiple sclerosis: a blinded, case-controlled study using a novel quantitative near-infrared spectroscopy method. Scientific Reports 2015;5:16477.
25. Varghese J, Potter LC, LaFountain R, Pan X, Raman SV, Ahmad R, Simonetti OP. CMR-based blood oximetry via multi-parametric estimation using multiple T2 measurements. J Cardiovasc Magn Reson 2017;19(1):88.
Figures