0063
Short and long sodium concentrations in multiple sclerosis: a multi-echo ultra- high field 23Na MRI study1Aix-Marseille Université, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie, Marseille, France, 3APHM, Hôpital de la Timone, Pôle d’Imagerie Médicale, CEMEREM, Marseille, France, 4Institute of Radiology, University Hospital Erlangen, Erlangen, Germany
Synopsis
Alteration of sodium homeostasis was previously evidenced in multiple sclerosis with total sodium concentration (TSC) found to be related to disability. However, the correlations found were moderate, maybe due to the fact that measured sodium accumulation combined intra and extra cellular sodium signal while only intra-cellular sodium concentration is relevant to assess neurodegeneration. One may suppose that developing reliable sequences able to assess only the intra-cellular signal may lead to a better estimation of neurodegeneration in multiple sclerosis and better correlations with irreversible disability. The present study proposes an original multi-TE sequence at 7T to reach this goal.
Introduction
Mitochondrial energy failure leads to axonal and neural degeneration in multiple sclerosis (MS) which is the main cause of irreversible disability1. This mitochondrial dysfunction results in intracellular sodium accumulation2, making. 23Na-MRI a promising biomarker of neurodegeneration in MS, which is lacking to date3. Thus, several studies were performed at 3T in multiple sclerosis and reported, as expected from pathological studies, significant and diffuse accumulations of total sodium concentration (TSC) that correlate with physical disability in patients with different phenotypes4–6. Nevertheless, only intra-cellular sodium accumulation is of interest to explore neurodegeneration2. To refine information provided by 3T 23Na-MRI, new MR contrasts have been proposed at higher field to separate the short fraction sodium signals (related to the intra-cellular compartment) and long fraction sodium signal (related to the extra-cellular)7-9. In the present study, we assessed the potential of multi-TE 23Na-MRI7 to asses sodium compartments accumulation in MS at an early stage of the disease at 7T and its correlations with clinical disability.Methods
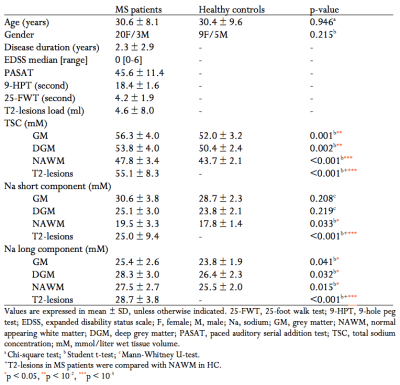
Subjects. Twenty-three relapsing-remitting MS patients at an early stage of the disease (20F, mean age 30.6±8.1years, disease duration 2.3±2.9years) and 14 healthy controls (HC) (9F, mean age 30.4±9.6years) were enrolled.Clinical examination. In all subjects, a clinical evaluation was performed including: expanded disability status scale (EDSS), paced auditory serial addition test (PASAT), 9-hole peg test (9-HPT) and 25-foot walk test (25-FWT).
7T MRI acquisition (Magnetom step2, Siemens). 23Na-MRI was acquired using a dual-tuned 23Na/1H birdcage coil (QED) and a multi-echo density adapted 3D projection reconstruction pulse sequence (TR=120ms, 10000 spokes, 384 radial samples/spoke, 3.0mm isotropic resolution, 24 echoes, TA=30min)7. To ensure a sufficient number and distribution of TEs while taking into account the 5ms readout of the sequence, we applied the sequence three times to obtain 24 TEs ranging from 0.20ms to 70.78ms. For quantification, six tubes with different sodium concentrations (25–100mmol/liter wet tissue volume (mM) in 2% agar) were placed within the FOV7. A high-resolution 1H 3D-MP2RAGE (TR/TE/TI1/TI2=5000/3/900/2750ms, 256 sagittal slices, 0.6mm isotropic resolution, TA=10min) was acquired using a 32-channel head coil (Nova).
3T MRI acquisition (Verio, Siemens). A 3D-MPRAGE and an axial T2-weighted sequence were acquired.
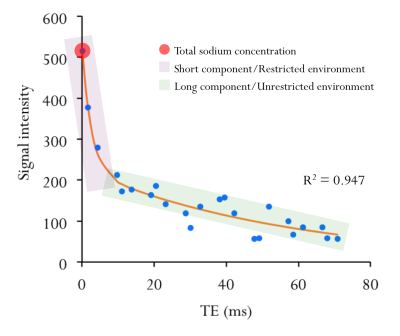
Data processing. White matter (WM) lesions were delineated on the 3T T2w images using an interactive semi-automated thresholding method10. T2w images were coregistered on the MPRAGE images using a rigid transformation11. MPRAGE and MP2RAGE images were skull stripped10,12 and coregistered using a non-linear transformation10. Lesions-filled13 MP2RAGE images were segmented into GM, WM (0.8 tissue probability threshold)14 and deep grey matter (DGM: accumbens, amygdala, caudate, hippocampus, pallidum, putamen and thalamus)15. Biexponential (Figure 1), monoexponential and linear fitting procedures were applied to the 23Na images to derive quantitative TSC map, 23Na short component (reflecting intra-cellular sodium concentration) and 23Na long component (reflecting extra-cellular sodium concentration)7. 23Na first echo images (TE=0.20ms) were registered on the MP2RAGE using a rigid transformation10. Linear and non-linear transformations were combined to bring T2-lesions mask to 23Na native space. Linear transformation was used to bring GM, WM and DGM masks to 23Na native space. Quantitative sodium maps (TSC, 23Na short and 23Na long) were quantified in GM, normal appearing white matter (masking for T2-lesions; NAWM), DGM and T2-lesions regions.
Statistical analysis. Data normality distribution was assessed using Shapiro-Wilk test. Comparisons between tissue types and groups were performed using Chi-square test, Mann-Whitney U-test and t-test. Spearman correlations were performed between TSC map, Na short and long components, T2-lesions volume and clinical scores (SPSS Inc, v. 23.0).
Results
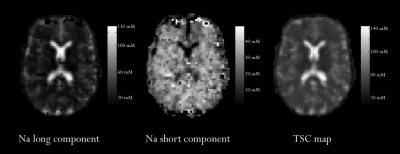
Demographical, clinical and MRI measures of our population are reported in Table 1. Figure 2 shows an example of quantitative sodium maps and the used regions of interest.In patients, TSC and 23Na short were significantly different between NAWM and T2-lesions (p<10-3 and 0.003, respectively). 23Na long in T2-lesions was higher than in NAWM without reaching a significant difference (p=0.050).
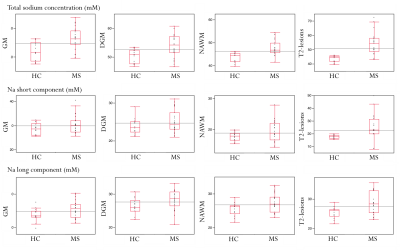
In MS patients compared to HC: TSC was significantly higher for GM (p=0.001), DGM (p=0.002), NAWM (p<10-3) and T2-lesions (p<10-3); 23Na short was significantly higher for NAWM (p=0.033) and T2-lesions (p<10-3); 23Na long was significantly higher for GM (p=0.041), DGM (p=0.032), NAWM (p=0.015) and T2-lesions (p<10-3) (Figure 3).
Regarding clinical scores, TSC in GM and NAWM were significantly correlated with 25-FWT (r=0.476, p=0.022 and r=0.567, p=0.004, respectively). 23Na short in NAWM was correlated with 25-FWT (r=0.469, p=0.024) and 23Na long in T2-lesions was correlated with 9-HPT (r=-0.464, p=0.026).
Discussion
The present study provides two main points. Firstly, total sodium concentration is higher in patients compared to controls and is correlated to clinical disability, which is in accordance to previous reports at lower field4–6. The main finding is the higher short and long component sodium concentration in T2-lesions and NAWM of patients compared to controls. As expected in this inflammatory disease, long component of sodium concentration reflecting interstitial edema is observed. Noteworthily, short component is also increased, suggesting that intra-axonal sodium accumulation significantly participate to TSC increase. Thus, these results confirms that neurodegeneration is partly reflected by TSC increase, as suggested by MRS studies16 or in neurodegenerative diseases such as ALS17. Moreover, these results are very promising for the use of multi-TE 23Na in assessment of intra-axonal sodium accumulation and subsequent neurodegeneration in brain diseases.Acknowledgements
This work was supported by the ANR grant JCJC ‘NEUROintraSOD-7T’ (ANR-15-CE19-0019-01) and the A*midex Imetionic-7 program.References
1. Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–S31.
2. Waxman SG. Mechanisms of Disease: sodium channels and neuroprotection in multiple sclerosis—current status. Nature Clinical Practice Neurology. 2008;4:159–169.
3. Inglese M, Oesingmann N, Zaaraoui W, Ranjeva JP, Fleysher L. Sodium imaging as a marker of tissue injury in patients with multiple sclerosis. Multiple Sclerosis and Related Disorders. 2013;2:263–269.
4. Zaaraoui W, Konstandin S, Audoin B, et al. Distribution of brain sodium accumulation correlates with disability in multiple sclerosis: a cross-sectional 23Na MR imaging study. Radiology. 2012;264:859–867.
5. Maarouf A, Audoin B, Pariollaud F, et al. Increased total sodium concentration in gray matter better explains cognition than atrophy in MS. Neurology. 2017;88:289–295.
6. Brownlee WJ, Solanky B, Prados F, et al. Cortical grey matter sodium accumulation is associated with disability and secondary progressive disease course in relapse-onset multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90:755–760.
7. Ridley B, Nagel AM, Bydder M, et al. Distribution of brain sodium long and short relaxation times and concentrations: a multi-echo ultra-high field 23Na MRI study. Scientific Reports [online serial]. 2018;8. Accessed at: http://www.nature.com/articles/s41598-018-22711-0. Accessed January 8, 2019.
8. Petracca M, Vancea RO, Fleysher L, Jonkman LE, Oesingmann N, Inglese M. Brain intra- and extracellular sodium concentration in multiple sclerosis: a 7 T MRI study. Brain. Epub 2016 Jan 20.:awv386.
9. Gilles A, Nagel AM, Madelin G. Multipulse sodium magnetic resonance imaging for multicompartment quantification: Proof-of-concept. Sci Rep. 2017;7:17435.
10. Maarouf A, Audoin B, Konstandin S, et al. Topography of brain sodium accumulation in progressive multiple sclerosis. Magn Reson Mater Phy. 2014;27:53–62.
11. Avants BB, Tustison NJ, Stauffer M, Song G, Wu B, Gee JC. The Insight ToolKit image registration framework. Front Neuroinform. 2014;8:44.
12. Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9:381–400.
13. Battaglini M, Jenkinson M, De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp. 2012;33:2062–2071.
14. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851.
15. Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922.
16. Donadieu M, Le Fur Y, Maarouf A, et al. Metabolic counterparts of sodium accumulation in multiple sclerosis: A whole brain 23 Na-MRI and fast 1 H-MRSI study. Multiple Sclerosis Journal. 2019;25:39–47.
17. Grapperon A-M, Ridley B, Verschueren A, et al. Quantitative Brain Sodium MRI Depicts Corticospinal Impairment in Amyotrophic Lateral Sclerosis. Radiology. 2019;292:422–428.
Figures