0058
A quantification of myelin and axonal damage across multiple sclerosis lesions and clinical subtypes with myelin and diffusion MRI1Translational Imaging in Neurology (ThINk) Basel, Department of Medicine and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 2Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 3Radiological Physics, Department of Radiology, University Hospital Basel, Basel, Switzerland, 4Department of Neurology, Lausanne University Hospital, Lausanne, Switzerland, 5Department of Radiology, Weill Cornell Medical College, New York, NY, United States, 6Department of Computer Science, University of Verona, Verona, Italy, 7Signal Processing Laboratory (LTSS), Ecole polytechnique fédérale de Lausanne (EPFL), Lausanne, Switzerland, 8Radiology Department, Center for Biomedical Imaging, Lausanne University and University Hospital, Lausanne, Switzerland, 9National Institute of Neurological Disorders and Stroke, Translational Neuroradiology Section, Division of Neuroimmunology and Neurovirology, Bethesda, MD, United States
Synopsis
The interplay between axonal and myelin damage in multiple sclerosis (MS) is poorly understood. This study aimed to evaluate the concomitant presence of axonal and myelin injury in living MS patients by using myelin and multi-shell diffusion MRI. Confirming neuropathological findings, our results show that (i) axonal and myelin damage exists in MS lesions and spreads out from the lesions in a centrifugal way, (ii) the extent of myelin and axonal damage differs among lesion subtypes and according to lesion anatomical locations and (iii) axonal (and not myelin) damage differs between relapsing-remitting and progressive MS patients.
Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system, which is characterized by myelin and axonal damage1. Neuropathological studies in postmortem brains showed axonal and myelin destruction in MS lesions and in normal-appearing (NA) brain tissue as well as variations in the extent of damage and repair among lesions at different locations2-6. Nevertheless, only few studies have addressed the complex interplay between myelin and axonal damage in living MS patients in small-sized relapsing-remitting MS (RRMS) cohorts7. In the current work, we have studied a large cohort of RRMS and progressive MS (PMS) patients and performed a comprehensive assessment of axonal and myelin damage in focal lesions and NA tissue. Specifically, we have evaluated axonal and myelin damage in: (1) cortical/white matter lesions (CLs, WMLs) and NA gray and white matter tissue (NAWM, NAGM); (2) peri-plaque WM; (3) lesions in different locations (periventricular vs juxtacortical) and lesions exhibiting ongoing chronic inflammation activity vs other lesions; (4) lesions in RRMS and PMS patients.METHODS
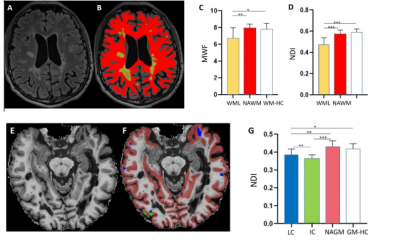
Sixty MS patients (41 RRMS and 19 PMS) and 35 healthy controls (HC) underwent multi-parametric magnetic resonance imaging (MRI). MRI was acquired in a 3T Prisma system (Siemens Healthcare, Germany) using a 64-channel head and neck coil. The MRI protocol included: (i) 3D FLAIR (TR/TE/TI=5000/386/1800 ms) and MP2RAGE (TR/TI1/ TI2=5000/700/2500 ms) with 1 mm3 isotropic spatial resolution; (ii) Myelin Water Imaging (TR/TE/resolution= 7.5 ms/0.54 ms/0.93*0.93*5 mm3); multi-shell diffusion (TR/TE/resolution= 4.5 s/75 ms/ 1.8 mm3 isotropic) and 3D EPI (TR/TE/resolution= 64 ms/ 35 ms/0.67*0.67*0.67 mm3).Myelin water fraction (MWF) and neurite density index (NDI) maps were reconstructed as in 8,9. Paramagnetic rims were identified on unwrapped phase images by 2 raters 10,11. Lesions were automatically segmented 12 and manually corrected. CLs were manually divided into intra-cortical and leuko-cortical lesions (ICLs and LCLs). Peri-ventricular (PV) and juxta-cortical (JC) lesions were defined as WM lesions located within 3mm from the boundary between WM and GM and WM and ventricles, respectively. We also manually segmented the mirror NA contralateral region of 200 WMLs and evaluated the percentage of myelin (%MWF) and axonal density (%NDI) reduction in WMLs.
MWF and NDI were extracted in (i) WMLs, CLs; (ii) two consecutive 2-voxel layers of peri-plaque WM (denoted as PP-1st and PP-2nd) ; (iii) NAWM and NAGM; (iv) WM and GM in HC (WM-HC and GM-HC) ; (v) PV and JC lesions ; (vi) paramagnetic rims lesions and non-rim lesions ; (vii) WMLs in RRMS and PMS patients.
Statistical analysis was performed by using nonparametric Mann-Whitney test (for two-group unpaired analyses) and Kruskal-Wallis test with Dunn’s correction for multiple comparison (for more than two-group analyses).
RESULTS
We analyzed 1310 MS lesions (WMLs: 1106, CLs: 204). In WMLs, MWF and NDI were reduced compared to NAWM and WM-HC (p< 0.001) (Figure 1 A-D). The %MWF reduction in WMLs compared to the mirror NAWM region was higher than the %NDI decrease (p< 0.0001). As to cortical lesions, ICLs showed lower MWF and NDI compared to NAGM (p< 0.01) whereas LCLs had lower NDI (p< 0.001) but equal MWF to NAGM (Figure 1 E-G). In PP-1st and PP-2nd of WMLs, MWF and NDI were significantly higher than in WMLs (p< 0.001) and lower than in WM-HC (p<0.001) (Figure 2). In PV lesions, MWF and NDI were lower than in JC lesions (p<0.0001) (Figure 3). In lesions with paramagnetic rims lesions, both MWF and NDI were lower compared with other WM lesions without paramagnetic rims (p<0.001) (Figure 4). NDI in lesions in PMS patients is lower than in RRMS (p<0.001) (Figure 5). However, the scatter plots (Figure 3&5) show that all WMLs exhibit a proportional decrease in myelin and axonal content.DISCUSSION
Confirming neuropathological findings, our study showed that WMLs and CLs exhibit significant myelin and axonal injury compared to normal-appearing and healthy tissue. In WMLs, myelin pathology outweighed axonal damage; in addition, diffuse axonal pathology was present - though at a lower extent - in peri-plaque NAWM. Adding to existing neuropathological knowledge, we also found that axonal damage in ICLs was higher than LCLs, which may indicate involvement of different pathogenic processes. Myelin pathology also differed according to lesion location and lesion type: PV lesions exhibited more axonal and myelin damage compared with JC lesions, as expected from previous neuropathological2-5 and PET-studies6; and paramagnetic rim lesions – which have been shown postmortem to exhibit extensive axonal damage in the center and ongoing damage in the surrounding tissue13 - exhibit as well more myelin and axonal reduction than lesions without rim. Our data also provide new knowledge about myelin and axonal damage in RRMS and PMS patients: axonal content in WMLs is lower in PMS than in RRMS whereas myelin damage is similar. This may well support the hypothesis that other mechanisms than demyelination lead to axonal damage in PMS14,15.CONCLUSION
Confirming neuropathological findings, our results in living patients show that (i) axonal and myelin damage exists in cortical and WM MS lesions as well as in peri-plaque WM and that (ii) the extent of myelin and axonal damage differs among lesion subtypes and between PVLs and JCLs. We have also provided new evidence that axonal (and not myelin) damage differs between RRMS and PMS.Acknowledgements
This work was funded by the Swiss National Funds PZ00P3_154508, PZ00P3_131914 and PP00P3_176984References
1.Rahmanzadeh R, et al. 2018. Multiple sclerosis pathogenesis: missing pieces of an old puzzle. Rev Neurosci. 2018; 30(1):67-83
2.Lucchenitti C, et al. 2000. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000.
3.Kutzelnigg A, et al. 2005. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain
4.Goldschmidt T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009; 72(22):1914-21.
5.Poiron E. Remyelination fails in the periventricular white matter in MS. ECTRIMS 2018
6. Bodini B, et al. 2016. Dynamic Imaging of Individual Remyelination Profiles in Multiple Sclerosis. Ann Neurol. 2016 May; 79(5): 726–738
7.Granberg T., et al. 2017. In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain
8.Nguyen TD, Deh K, Monohan E, et al. Feasibility and reproducibility of whole brain myelin water mapping in 4 minutes using fast acquisition with spiral trajectory and adiabatic T2prep (FAST-T2) at 3T. Magn Reson Med. 2016; 76(2):456-65.
9. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000-1016
10.Maggi P. et al. 2019. Paramagnetic phase rims and serum neurofilaments in relapsing-remitting and progressive multiple sclerosis patients: a combined laboratory-imaging marker of chronic inflammation. ECTRIMS 2019.
11.Absinta M, et al. Identification of Chronic Active Multiple Sclerosis Lesions on 3T MRI. AJNR 2018.
12.Fartaria MJ et al. Partial volume-aware assessment of multiple sclerosis lesions. Neuroimage Clin.2018
18: 245–253. 13. Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7T magnetic resonance imaging. Acta neuropathologica. 2016
14.Faissner S.et al. 2019. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov. 2019 Aug 9
15.Lassmann H. et al. 2012. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012 Nov 5;8(11):647-56.
Figures