0055
On the Potential of Whole-Brain Postmortem MR Imaging at 3T: New Insights into Multiple Sclerosis with Resolutions Up to 200μm1Translational Imaging in Neurology (ThINk) Basel, Department of Medicine and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 2Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 3Radiological Physics, Department of Radiology, University Hospital Basel, Basel, Switzerland, 4Department of Cognitive Neurology, MR-Research in Neurology and Psychiatry, University Medical Center Göttingen, Göttingen, Germany, 5Institute of Neuropathology, University Medical Center Göttingen, Göttingen, Germany, 6Translational Neuroradiology Section, Division of Neuroimmunology and Neurovirology, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States
Synopsis
MR imaging is an indispensable tool for the depiction of human brain anatomy and pathology. Besides in vivo acquisitions, MRI of the fixated human brain is highly interesting: Very long scan times basically allow unprecedented MRI resolutions on clinical scanners. The present work describes an MRI approach that was developed for standard clinical 3T systems and tests for the viable boundaries: Within scan times between a few hours up to a weekend, acquisitions of high soft tissue contrast with isotropic resolutions up to 200μm can be achieved; revealing fine structure details and allowing an impressing lesion detection and characterization.
Introduction
Postmortem (PM) MR imaging of the ex vivo healthy and diseased human brain offers the great potential for a deeper understanding of neuro morphology and pathology, including valuable comparisons with histopathological analysis1,2. The lack of many sources of artifacts and possible scan times within the range of ~days facilitates MR imaging with resolutions and of a quality not achievable for in vivo scans.Like in a recent experiment achieving even a 100μm resolution3, PM whole-brain imaging is frequently performed at 7T field strength, since it generally provides higher SNR per unit time, which is the most limiting factor in this case in terms of achievable resolution. 3T MR systems, however, have the benefit of being much more widely available, are more economic in operation, they provide quite homogeneous excitation (B1+) fields, and, finally, the acquired PM data allow more direct comparisons with patient data from clinical routine.
The purpose of this work was to develop and to test for the viable boundaries of a very-high resolution MR acquisition approach with a strong soft tissue contrast, dedicated for whole-brain PM imaging that can be achieved on a modern, but standard hardware 3T MR system, acquired within the “economical time of a weekend”.
Methods
Brain preparation and Experimental setupThe brain of a patient with secondary progressive MS was fixated in 10% formalin approx. 36h after autopsy. For MRI acquisition, the brain was positioned in a dome-shaped container as depicted in 4-6 and immersed in Fomblin, a fluorinated oil. Air bubbles were aspirated through the spout of the container through a vacuum pump. All acquisitions were performed with a 3T wholebody MR system (MAGNETOM PrismaFit, Siemens Healthcare, Erlangen, Germany) using the standard 20-channel phased-array head coil.
Acquisition
Based on our experience that, basically, multi-gradient-echo sequences harnessed for quantitative susceptibility mapping7 allow to acquire high-quality PM images under stable signal conditions, an RF spoiled gradient echo sequence was taken as the initial basis. To circumvent the limitations of vendor sequences here regarding, e.g., maximal 3D matrix size – they are usually optimized for clinical in vivo applications – a corresponding in-house sequence with a strong 4π per voxel spoiler moment and RF spoiling was programmed. As an additional benefit, a more precise monitoring of the required MR system performance was feasible.
Three different base protocols with isotropic resolutions of 200μm, 240μm, and 270μm were set up. All experiments used a constant FOV of 192x192mm2. Protocol specific MR parameters are depicted in Table 1. This table also informs about repeated acquisitions for later averaging.
To exploit the fast T1 recovery for strong signal generation, the excitation flip angle was selected close to the Ernst angle8; however, was chosen a few degrees higher to enhance tissue contrast. A low bandwidth of 80Hz/Px was employed to further enhance the SNR and to maximize the time spent for signal acquisition for given TE and TR.
For image reconstruction, solely the standard MR system reconstruction was used: no kind of filtering, no interpolation, absolutely no image registration concepts.
Results
Figure 1 compares acquisitions with the three base protocols depicted in Table 1. These were visually perceived as providing sufficient SNR in a minimum time. Even detailed structures like the basal ganglia are resolved very nicely, a good signal homogeneity is provided as well.Based on the 270μm base protocol, Figure 2, left part, presents the gain in SNR to be expected by means of (here manual) averaging the images of repeated measurements (Table 1). The right part further illustrates the benefit of a coronal reformation (upper row) and also contrasts the “best” 200μm resolution acquisition (right part, lower row). As can be also deduced from these acquisitions, our complete setup is stable enough for measuring 200μm to 270μm resolutions over up to 54h (Table 1).
Figure 3 shows the stria of Gennari in the occipital lobe at both 270μm and 200μm resolution.
Figure 4 shows different small-sized multiple sclerosis lesions (intracortical and juxtacortical): we could describe for the first time lesion with a paramagnetic rim extending to both WM and cortical lesion areas (Figure 3 A,I); and others where the rim was only surrounding the white matter part of the lesions (Figure 3 B,C).
Discussion and Conclusion
A postmortem MRI approach was developed that facilitates isotropic resolutions up to 200μm with a strong soft tissue contrast. Compared to recent 7T studies3 that use a similar acquisition sequence approach, our development is dedicated to any standard 3T MRI system regarding hardware performance and components (gradient strength 40mT/m only, 20ch head coil) and does not need any hardware modifications. Even the “low” 270μm base protocol offers great potential for a 5.5h acquisition time; a protocol that can be easily attached to other postmortem examinations.As demonstrated, the full capabilities like a 200μm acquisition are revealed for our PM approach covering a full weekend, which can be usually organized well in the light of routine measurements at site. First clinical results depicted in Figures 3 and 4 underline the impact of 3T based PM examinations on basic and clinical neuro science.
Acknowledgements
This work was funded by the Swiss National Funds PZ00P3_154508, PZ00P3_131914 and PP00P3_176984.References
1. Pfefferbaum A, Sullivan EV, Adalsteinsson E, et al. Postmortem MR imaging of formalin-fixed human brain. Neuroimage 2004;21(4):1585-95.
2. Miller KL, Stagg CJ, Douaud G, et al. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage 2011;57(1):167-181.
3. Edlow B, Mareyam A, Horn A, et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data. 2019 Oct 30;6(1):244.
4. Luciano NJ, Sati P, Nair G, et al. Utilizing 3D Printing Technology to Merge MRI with Histology: A Protocol for Brain Sectioning. J Vis Exp 2016 Dec 6;(118).
5. Absinta M, Nair G, Filippi M, et al. Postmortem Magnetic Resonance Imaging to Guide the Pathologic Cut: Individualized, 3-Dimensionally Printed Cutting Boxes for Fixed Brains. J Neuropathol Exp Neurol. 2014;73(8):780-8.
6. Griffin AD, Turtzo C, Parikh G, et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019;142(11):3550-3564.
7. Spincemaille P, Anderson J, Wu G, et al. Quantitative Susceptibility Mapping: MRI at 7T versus 3T. J Neuroimaging 2019. doi: 10.1111/jon.12669. [Epub ahead of print]
8. Ernst RR. Application of Fourier transform spectroscopy to magnetic resonance. Review of Scientific Instruments 1966;37: 93.
Figures
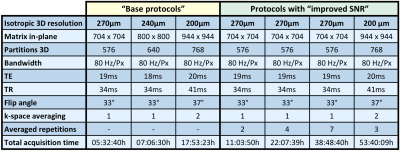
Table 1: Comparison of important protocol parameters for the three resolutions. The table distinguishes between two types of signal averaging for improving SNR, which are standard in MRI:
(1) Directly averaging k-space lines within one acquisition (also called “inner averaging”), and
(2) manual averaging of the acquired magnitude images from repeated base protocol measurements - as specified.

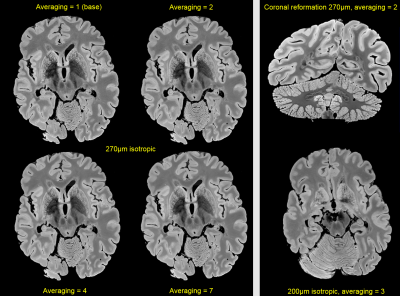
Figure 2: Left side: Visual SNR-gain by averaging repeated measurements. The SNR only improves with the square root of averages, but the acquisition time scales linearly (Table 1). Thus in terms of ‘efficiency’, it may be worthwhile to limit the number of averages. Right side: (Upper row) Based on exploiting the full isotropic resolution, valuable coronal reformations can be made. (Lower row) For comparison, a different slice acquired with the “best” 200μm resolution is shown (for more overview).
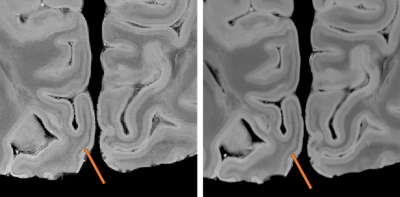
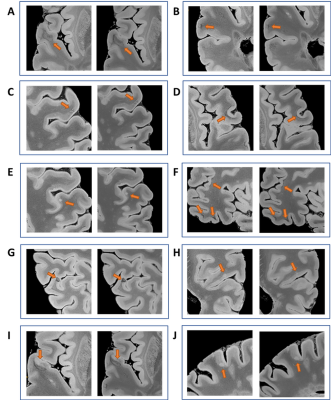
Figure 4: High-magnification patches of different type of small MS lesions that will be missed with lower spatial resolution; 200μm (left) to 270μm (right). Leuco-cortical lesions (A-D, H-I) and intracortical lesions (E,G,J); juxtacortical siderosis: (F).
Interestingly, for the first time we describe intracortical lesions with paramagnetic rims (G) and lesions with paramagnetic rims that are covering only the WM part of leuco-cortical lesions (A,I) or the entire leuco-cortical lesion (B,C).