0054
Longitudinal changes of myelin water fraction during the first year after moderate to severe diffuse traumatic brain injury1Epilepsy Center / Neurological Institute, Cleveland Clinic, Cleveland, OH, United States, 2Moss Rehabilitation Research Institute, Elkins Park, PA, United States, 3Department of Molecular, Cellular, and Biomedical Sciences, CUNY School of Medicine, The City College of New York, New York, NY, United States, 4Division of Biomedical Engineering, Hankuk University of Foreign Studies, Yongin, Korea, Republic of, 5Laboratory for Imaging Science and Technology, Department of Electrical and Computer Engineering, Institute of Engineering Research, Seoul National University, Seoul, Korea, Republic of
Synopsis
Reliable MRI biomarkers of white matter degeneration can be useful for monitoring post-traumatic progressive neurodegeneration and identifying potential treatment targets. We report that myelin water signal can be measured reliably during the first year after moderate to severe traumatic axonal injury. We also report that apparent myelin water fraction from the whole brain white matter continued to decrease beyond 3 months post-injury, reflecting progressive axonal degeneration and demyelination.
INTRODUCTION
Traumatic axonal injury (TAI), one of the signature consequences of traumatic brain injury (TBI), triggers a cascade of events that lead to neurodegeneration. Diffusion tensor imaging (DTI) has been widely used during the past two decades to assess white matter integrity after TAI1. Longitudinal DTI studies thus far revealed both increase and decrease over time of DTI metrics including fractional anisotropy (FA)2-4. However, clear interpretation of these findings is hampered by the facts that DTI metrics are inherently limited in distinguishing different types of white matter pathologies, and that they are likely to be influenced by multiple factors including crossing fibers, vasogenic edema and chronic neuroinflammation. We have recently shown that myelin water imaging (MWI) using the direct visualization of short transverse component (ViSTa) method can be used as an alternative biomarker of white matter integrity after TAI5. In the current study, we expand our prior findings by examining longitudinal changes in myelin water signal over the first year after moderate to severe TAI.METHODS
Subjects: Fifteen individuals with moderate to severe TBI were scanned at 3, 6, and 12 months post-injury for longitudinal changes in myelin water signal. In addition, 30 demographically matched healthy controls (HC) were scanned once.ViSTa-MWI: A Siemens Trio 3T MRI scanner was used for data acqusition. A 3D segmented EPI based ViSTa sequence6 was implemented using the following parameters: resolution = 1.38x1.38x5 mm3, 26 slices, TR/TE = 1160/4.5 ms, TI1/TI2/TD = 560/220/380 ms, partial k-space = 6/8, EPI factor = 15, and scan time = 7 min 33 sec. To quantify apparent myelin water fraction (aMWF), reference scan, which is a proton density weighted gradient echo sequence based on the same EPI as the ViSTa sequence, was acquired (TR = 97 ms, flip angle = 28°, and scan time = 38 sec). The aMWF was calculated by dividing the ViSTa data by the reference data and multiplying a scaling factor6.
Data processing: To calculate aMWF in white matter across the whole brain, all ViSTa MWI images from HC and TBI patients at all time points were registered non-linearly to a JHU FA map using SyN in ANTs7,8. A white matter mask was generated by thresholding a JHU FA map. The voxels that were one standard deviation smaller than the mean value of the JHU FA map were excluded in the white matter mask. aMWF across voxels in the white matter mask was averaged for subsequent analysis.
Statistical analysis: Spearman’s correlation coefficients among 3-, 6-, and 12-month assessments in TBI patients were calculated. The Mann-Whitney U-test was used to evaluate the statistical difference between TBI and HC groups. To explore longitudinal changes in aMWF within the TBI group, aMWF values at each time point were compared with each other using a Wilcoxon signed-rank test. To evaluate the clinical relevance of aMWF, Spearman’s correlation coefficient was calculated between aMWF and the duration of post-traumatic amnesia (PTA).
RESULTS
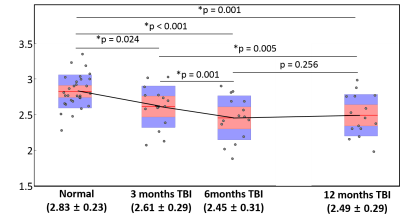
Strong correlations were found among aMWF values at each scan sessions (between 3- and 6-month scans: rho = 0.914 and p < 0.001, between 3- and 12-month scans: rho = 0.911 and p < 0.001, and between 6- and 12-month scans: rho = 0.911 and p < 0.001).Figure 1 shows longitudinal aMWF values at 3, 6, 12 month post-injury in TBI patients with those of matched HC. Compared to HC, the patient group showed significantly reduced aMWF at all time points (7.8% reduction and p = 0.024 at 3-month follow-up, 13.4% reduction and p < 0.001 at 6 month, and 12.0% reduction and p = 0.001 at 12 month). Within the TBI patient group, significant changes were detected between 3- and 6-month post-injury aMWF values (6.1% decrease; p = 0.001) and between 3- and 12-month (4.6% decrease; p = 0.005), but there was no significant difference between 6- and 12-month scans (p = 0.256).
Regarding the relationship between aMWF and PTA, significant correlations were found at 3 months (rho = -0.617; p = 0.014) and 12 months (rho = -0.535; p = 0.040). The correlation at 6 months post-injury was marginally significant (rho = -0.509; p = 0.052).
DISCUSSION
Conventional DTI cannot determine whether disruptions in DTI metrics are due to axonal membranes, myelin sheath, or other components of microstructure. The current study demonstrated, although in a small sample, that myelin water signal can be measured reliably during the first year after moderate to severe TAI. We also report that aMWF from the whole brain white matter continued to decrease beyond 3 months post-injury, reflecting progressive axonal degeneration and demyelination. Future studies with a larger sample, more frequent time points, and region-of-interest analysis using ViSTa MWI are warranted to reveal the spatial and temporal dynamics of longitudinal white matter degeneration after TAI.CONCLUSION
Longitudinal measurement of myelin water using ViSTa MWI may serve as a reliable biomarker of white matter integrity through the course of progressive axonal degeneration and demyelination after TAI.Acknowledgements
This study was supported by an NIH (NINDS) grant 5R01NS065980 (PI:
JJK).
References
1. Wallace EJ, Mathias JL, and Ward L. Diffusion tensor imaging changes following mild, moderate and severe adult traumatic brain injury: a meta-analysis. Brain Imaging and Behavior. 2018;12(6):1607-1621.
2. Farbota KD, Bendlin BB, Alexander AL, et al. Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front Hum Neurosci. 2012;6:160.
3. Kumar R, Saksena S, Husain M, et al. Serial changes in diffusion tensor imaging metrics of corpus callosum in moderate traumatic brain injury patients and their correlation with neuropsychometric tests: a 2-year follow-up study. J Head Trauma Rehabil. 2010; 25(1): 31–42.
4. Newcombe V, Correia M, Ledig C, et al. Dynamic changes in white matter abnormalities correlate with late improvement and deterioration following TBI. Neurorehabil Neural Repair. 2016;30(1):49-62.
5. Choi J, Hart T, Whyte J, et al. Myelin water imaging of diffuse traumatic brain injury. Neuroimage Clin. 2019;22:101785.
6. Oh SH, Bilello M, Schindler M, et al. Direct visualization of short transverse relaxation time component (ViSTa). Neuroimage. 2013;83:485-492.
7. Avants B and Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage. 2004;23 Suppl 1:S139-150.
8. Avants BB, Yushkevich P, Pluta J, et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage, 2010;49(3):2457-2466.