0053
Quantitative myelin imaging in the ferret brain for traumatic brain injury research1University of Arizona, Tucson, AZ, United States, 2Vanderbilt University, Nashville, TN, United States
Synopsis
MRI tools for mapping myelin content could provide useful markers of injury and repair in neurologic disorders that preferentially affect white matter such as traumatic brain injury (TBI). In this study, we apply two novel myelin water mapping approaches - bound pool fraction (BPF) from selective inversion recovery MRI and myelin water fraction (MWF) from multiple spin echo MRI - in the ex-vivo ferret brain with and without injury in order to develop these myelin mapping markers for pre-clinical TBI research. We demonstrate high quality BPF and MWF maps and describe metric behavior in a region of focal injury.
Introduction
White matter damage or disruption is associated with numerous brain disorders and is a prominent pathology in traumatic brain injury (TBI). Because white matter injury can arise from a variety of different cellular changes – e.g. axonal injury/degeneration, demyelination, gliosis, etc. – and each can have different consequences and treatment targets, it would be advantageous to develop imaging tools with specificity for different white matter pathologies in order to provide distinct markers both for clinical use and also for brain research in pre-clinical (i.e. animal model) studies. Markers of myelin damage are particularly important for identifying and following white matter alterations after experimental TBI and the development of myelin imaging tools will enable a more complete understanding of the time course and pathomechanisms of secondary injury and could provide more sensitive markers for the detection of mild injury which is a major challenge in TBI research and diagnosis. Advances in myelin water fraction (MWF) and bound pool fraction (BPF) mapping techniques both in acquisition and modeling are promising for advancing these goals. The objective of this work was to implement and optimize two novel strategies for myelin water imaging – selective inversion recovery (SIR) for BPF mapping(Gochberg and Gore, 2007) and multi-echo T2 for MWF mapping(Prasloski et al., 2012) – in the ferret brain. Through high resolution ex-vivo imaging, this work was able to evaluate methodologic aspects of implementing these strategies, compare them directly and begin to identify markers of pathology in a TBI model.Methods
Ex-vivo ferret brains (n=4) with and without TBI were obtained from an existing diffusion MRI study of closed head rotational injury and imaged using a Bruker 7T MRI scanner with a 35mm quadrature coil and running Paravision 360 software. The ferret was selected for this study based on its high white matter volume (relative to rodents), which is advantageous for reducing partial volume effect.Two myelin water mapping techniques were performed using pulse sequences and scan protocols adapted for Paravision 360 scanner software as well as modeling software ( https://remmi-toolbox.github.io) to fit the data and generate BPF and MWF metrics. All scans were collected using 3D acquisition with 250 micron isotropic resolution.
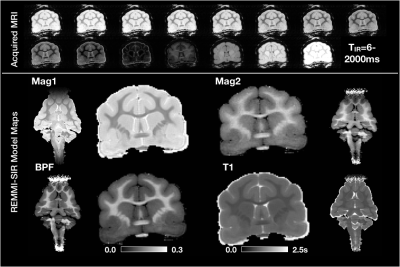
For BPF mapping, a selective inversion recovery (SIR) acquisition was used with TE/TR=6/1590ms and 15 different inversion recovery times from 6-1500ms with logarithmic spacing (see figure 1). Total scan time was 11h:45m.
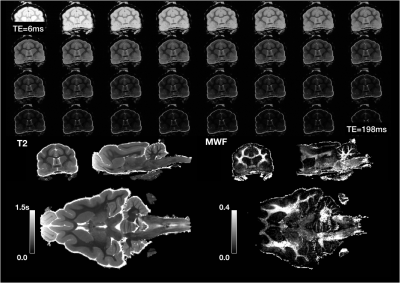
For MWF mapping, a multi spin echo RARE pulse sequence was used with 32 different echo times of TE=6-198ms and TR=5000ms. Total scan time was 6h.
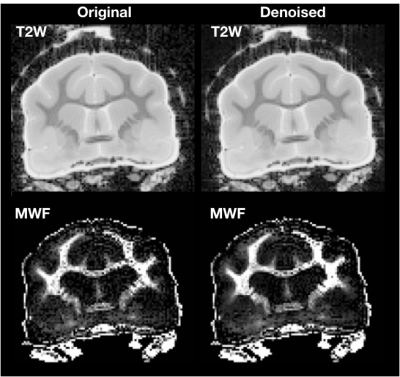
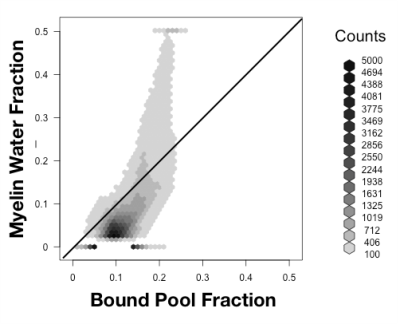
Both models were fit using the REMMI software toolbox in Matlab (R2019b, Natick, MA, https://remmi-toolbox.github.io). For denoising of the T2 data a random matrix tool for dwi-denoisng was used(Veraart et al., 2016). In order to directly compare BPF and MWF, value pairs were counted in a 2D histogram for all voxels in the brain using the hexbin package of the R statistical package.
Results and Discussion
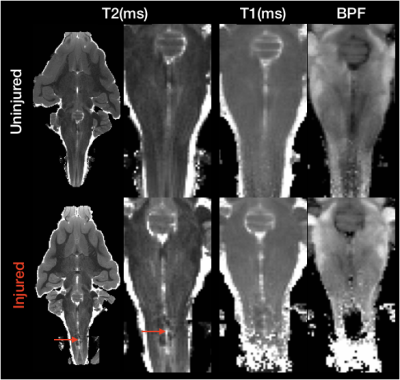
High quality images were collected using the REMMI pulse sequences and the ex-vivo ferret specimens with SNR values for the MSE of 363 (TE=6) to 22.4 (TE=198ms) and up to 137.8 for the SIR acquisition. This resulted in quantitative maps for T2, T1, BPF and MWF of good quality although the dependence on SNR was very apparent in the region of signal drop off at the end of coil coverage. The MWF maps appeared to have the greatest vulnerability to noise of all maps, which denoising of the raw data was able to alleviate partially (Figure 3). Comparison of BPF and MWF values in the ferret brain showed that in the lower range of bound fraction (<0.15) likely corresponding with gray matter, BPF values were higher than MWF but at high values likely corresponding with white matter, the metric relationship was more direct or MWF values were greater than BPF values. This relationship may reflect biological underpinnings, but could also be influenced by different noise dependence and should be explored in more detail by future work. Finally, the observation of BPF in a region of injured spinal cord tissue and comparison with T1 and T2 maps suggests a striking and well delineated reduction of BPF in the region of abnormality with a distinct spatial pattern from the T1 and T2 maps. If this decreased BPF indeed reflects the loss of myelin in the injured region, it would be a potentially useful and specific marker for an important facet of TBI pathology.Acknowledgements
The authors are grateful for the funding that makes this possible including the NIH grant EB019980 (KH and MD) for the development and distribution of the MRI acquisition and modeling tools and funding from the Center for Neuroscience and Regenerative Medicine for the ferret TBI work as well as the quantitative medical imaging section at the NIBIB and Carlo Pierpaoli. MRI scanning was supported by the Translational Bioimaging Resource at the University of Arizona.References
Gochberg DF, Gore JC (2007) Quantitative magnetization transfer imaging via selective inversion recovery with short repetition times. Magn Reson Med 57:437-441.
Prasloski T, Madler B, Xiang QS, MacKay A, Jones C (2012) Applications of stimulated echo correction to multicomponent T2 analysis. Magn Reson Med 67:1803-1814.
Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E (2016) Denoising of diffusion MRI using random matrix theory. NeuroImage.
Figures