0037
Developing a Universally Useful, Useable and Used Standardized MRI Protocol for Patients with Multiple Sclerosis1Radiology, University of British Columbia, Vancouver, BC, Canada, 2VU University Medical Center, Amsterdam, Netherlands, 3Johns Hopkins University, Baltimore, MD, United States, 4Consortium of MS Centers, Hackensack, NJ, United States, 5Children's Hospital of Philadelphia, Philadelphia, PA, United States, 6UBC MRI Research Center, University of British Columbia, Vancouver, BC, Canada, 7National MS Society, New York, NY, United States, 8Multiple Sclerosis Association of America, Cherry Hill, NJ, United States, 9Cortechs Labs, San Diego, CA, United States, 10London MS Clinic, Western University, London, ON, Canada, 11University of Toronto, Toronto, ON, Canada, 12NeuroCure Clinical Research Center, Charité Universitätsmedizin, Berlin, Germany, 13General Electric Healthcare, Milwaukee, WI, United States, 14Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, MD, United States, 15University of British Columbia, Vancouver, BC, Canada, 16University of Pennsylvania, Philadelphia, PA, United States, 17icometrix, Leuven, Belgium, 18BIU MR, Philips Healthcare, Eindhoven, Netherlands, 19VA MS Medical Center of Excellence-East, Washington, DC, United States, 20McGovern Medical School, University of Texas Health Science Center, Houston, TX, United States, 21Neurology, University of British Columbia, Vancouver, BC, Canada
Synopsis
Standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis (MS) have been available for over a decade. These guidelines are useful and useable, but not yet widely used. An expert panel with representatives from CMSC, NAIMS, NMSS, MSAA and MRI vendors updated the MRI protocol with the vision of creating international guidelines to be universally adopted as the standard of care for use of MRI in MS. Novel methods of disseminating information to payers, patient groups, MRI Centers and MRI vendors so that the MRI protocol will be used were discussed and will be explored.
INTRODUCTION
Magnetic resonance imaging (MRI) is of great value in the diagnosis and follow-up of patients with multiple sclerosis (MS). Experts agree that a standardized examination is key to the accurate identification of new lesions and activity which permits earlier diagnosis and prompts initiation and/or change in disease modifying therapy. Presently, there are two recommended protocols: Guidelines from the Consortium of Multiple Sclerosis Centers (CMSC) in North America and the Magnetic Resonance Imaging in MS group (MAGNIMS) in Europe.1-5 While these guidelines are recognized as being extremely useful and increasingly useable, they have not yet been widely used and adopted.METHODS
The CMSC convened an expert panel on October 25, 2019 to update the guidelines for a standardized MRI protocol for the diagnosis and follow-up of MS which were first developed in 2001 and subsequently updated 5 times. In addition to updating the guidelines, a major aim of the meeting was to discuss and develop plans to promote its use. Conference attendees consisted of neurologists, radiologists, MR technologists and imaging scientists with an expertise in MS from the United States, Canada and Europe, including representatives from CMSC, MAGNIMS, NAIMS (North American Imaging in Multiple Sclerosis Cooperative), National MS Society (NMSS), MS Association of America (MSAA), and representatives from leading MRI manufacturers and commercial image analysis companies. Prior to the meeting, the CMSC also surveyed its members regarding the use of MRI with a consistent standardized protocol, as well as for the use of gadolinium, diffusion weighted imaging sequences in MS diagnosis and follow-up, and the central vein sign for diagnosis.RESULTS and DISCUSSION
The CMSC MRI protocol was updated (Figures 1, 2, 3).The critical importance of using the subcallosal plane for consistent repositioning of axial oblique slices was again highlighted. Auto-prescription tools may be helpful for less experienced centers. The requirement for thin (< 3mm), non-gapped slices was re-emphasized.
For the Brain (Figure 1), in addition to the Core (mandatory for all MRI studies; axial and sagittal FLAIR and axial T2), Gadolinium (when use of gadolinium is indicated) and Additional (optional) sequences that had been detailed with the previous guidelines, there was group consensus for a new category of Optimum Plus sequences, which allow for quantitative brain volume measurements (3D high resolution T1) and the assessment of the central vein sign and chronic active rim lesions (susceptibility weighted sequence). These were highly recommended for their potential usefulness but could not be designated Core sequences as they were not in routine use for all clinical scanners either because of sequence availability or they were not used because the additional scan time required could not be justified for the potential value of the sequence.
For progressive multifocal encephalopathy (PML) surveillance, an abbreviated protocol with only FLAIR and DWI was recommended (Figure 2).
For the Spine (Figure 3), as centers vary in their use and experience with different sequences, it was recommended that centers acquire 2 of the 4 recommended sequences in the sagittal plane.
While there is still no known CNS toxicity of gadolinium deposition (which occurs for all agents but to a much lesser degree with macrocyclic agents), gadolinium should be used judiciously, recognizing its continued important role in MS. It is invaluable in patients presenting with their first attack (so called 'clinically isolated syndrome') as it allows for an earlier diagnosis by demonstrating disease dissemination in time. It is also essential for following patients with highly active disease, when there is unexplained and unexpected clinical worsening and when there is concern regarding an alternative diagnosis other than MS. Gadolinium use however is not necessary for routine follow-up to detect sub-clinical disease which could lead to a change in therapy, because new lesion activity can be easily identified on well-performed MRI using a standardized protocol, unless there is a large T2 burden.
In the process of updating the protocol there was a consistent effort to make the CMSC and MAGNIMS MRI protocols similar so that the updated protocol could eventually be presented to MAGNIMS for review and revision (if necessary) and ultimately viewed as the international consensus MRI protocol. There was enthusiasm for the MRI vendors to incorporate a single MS (or ‘white matter’) protocol on all their scanners. Other methods for dissemination were discussed including Webinars and developing cards with the MRI protocol for distribution to clinicians, radiologists, MS & MRI centers as well as patients. In addition, discussions will be planned with third party payers and insurance companies on the benefit of a standardized MRI examination versus the waste of a scan that cannot be compared with a prior (or future) study because of technical differences. Quality improvement projects would also help to assess the impact of having (and not having) a consensus protocol on practice and standard of care.
CONCLUSION
The expert group convened recently by the CMSC has updated the MRI guidelines with the vision and action plans for the MRI protocols to be universally useful, useable and used as the standard of care for patients with MS.Acknowledgements
No acknowledgement found.References
1. Traboulsee A, Simon JH, Stone L, et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol. 2016;37(3):394-401.
2. The Consortium of MS Centers MRI Task Force. Consortium of MS Centers MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-up of MS. 2018 Revised Guidelines. 2018. https://cdn.ymaws.com/mscare.site-ym.com/resource/collection/9C5F19B9-3489-48B0-A54B-623A1ECEE07B/2018MRIGuidelines_booklet_with_final_changes_0522.pdf. Accessed November 4, 2019.
3. Rovira À, Wattjes MP, et al, on behalf of the MAGNIMS study group. MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471-482.
4. Wattjes MP, Rovira À, et al,on behalf of the MAGNIMS study group. MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11(10):597-606.
5. Arevalo O, Riascos R, Rabiei P, Kamali A, Nelson F. Standardizing Magnetic Resonance Imaging Protocols, Requisitions, and Reports in Multiple Sclerosis: An Update for Radiologist Based on 2017 Magnetic Resonance Imaging in Multiple Sclerosis and 2018 Consortium of Multiple Sclerosis Centers Consensus Guidelines. J Comput Assist Tomogr. 2019;43(1):1-12.
Figures
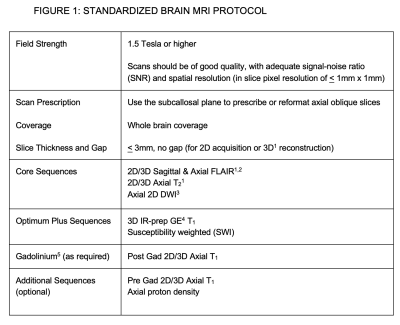
Figure 1: Standardized Brain MRI Protocol
1 3D acquisition voxel size isotropic < 1x1x1mm or over-contiguous acquisition, slice thickness < 1.5 mm and overlap < 0.75 mm
2 FLAIR (Fluid Attenuated Inversion Recovery)
3 DWI (Diffusion Weighted)
4 IR-prep GE (Inversion-recovery prepared Gradient Echo); Magnetization Prepared Rapid Acquisition Gradient Echo or MP-RAGE; Turbo Field Echo or TFE
5 Single dose of gadolinium-based contrast agent (FLAIR or T2 may be performed during the 5 minute minimum delay after gadolinium injection before acquiring the post-gadolinium T1)
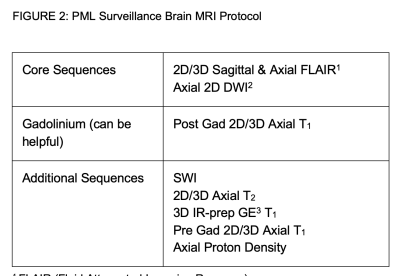
Figure 2: PML Surveillance Brain MRI Protocol
1 FLAIR (Fluid Attenuated Inversion Recovery)
2 DWI (Diffusion Weighted)
3 IR-prep GE (Inversion-recovery prepared Gradient Echo); Magnetization Prepared Rapid Acquisition Gradient Echo or MP-RAGE; Turbo Field Echo or TFE
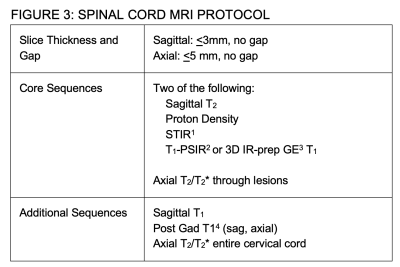
Figure 3: SPINAL CORD MRI PROTOCOL
1 STIR (Short Tau Inversion Recovery)
2 PSIR (Phase Sensitive T1 Inversion Recovery)
3 IR-prep GE (Inversion-recovery prepared gradient Echo); Magnetization Prepared Rapid Acquisition Gradient Echo or MP-RAGE; Turbo Field Echo or TFE
4 No additional gadolinium necessary if cord examination immediately follows gadolinium enhanced brain MRI