0033
Water exchange across blood-brain barrier is associated with CSF Amyloid-β 42 and cognition in healthy older adults1Mark & Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Department of Neuroscience, College of Medicine, University of Kentucky, Lexington, KY, United States, 3Department of Neurology, University of Southern California, Los Angeles, CA, United States
Synopsis
Abnormally low CSF amyloid-β (Aβ)-42 level is an early biomarker of the Alzheimer’s Disease (AD), however lumbar puncture is required to collect CSF samples. A diffusion prepared arterial spin labeling technique was proposed to measure blood-brain barrier (BBB) water permeability (kw) non-invasively. Associations between water permeability and CSF Aβ42 levels in the “healthy” aging brains were studied. Significant positive correlations were found between kw and CSF Aβ42 and digit symbol scores, which suggests kw may serve as an early imaging marker of AD.
Introduction
Abnormally low CSF amyloid-β (Aβ)-42 level is an early biomarker of the Alzheimer’s Disease (AD) associated pathologic changes in the brain, however lumbar puncture is required to collect CSF samples.1 In the CNS, water exchange across the blood-brain barrier (BBB) is mainly facilitated by water channel protein aquaporin-4 (AQP4). Accumulating evidence suggests that AQP4 is important to maintain glymphatic functions and assists clearance of deleterious proteins including Aβ.2,3 We proposed a novel diffusion-prepared 3D gradient and spin echo (GRASE) pseudo-continuous arterial spin labeling (pCASL) sequence to non-invasively measure water permeability across the BBB with good test and retest reproducibility,4 which could provide a direct and sensitive measurement of BBB integrity and AQP4 function. The purpose of this study was to study the associations of water permeability and CSF Aβ42, tau and cognition levels in the “healthy” aging brains.Methods
The MR pulse sequence design of diffusion prepared 3D GRASE pCASL has been detailed previously.4 Imaging parameters were: FOV = 224 mm, matrix size = 64×64, 12 slices (10% oversampling), resolution = 3.5×3.5×8 mm . A 2-stage approach was employed to measure cerebral blood flow (CBF), arterial transit time (ATT) and water exchange rate across the BBB (kw) in 9 mins 20 sec.5 Intra-/extra vascular components of perfusion signal can be separated by a small diffusion gradient, and kw was quantified from ATT and intra-/extra-vascular ratio, using a total generalized variation (TGV) regularized single-pass approximation (SPA) model.4Thirty-nine older adults (20 female, age=72.7±5.3 yrs) without neurological disease underwent MRI on a Siemens 3T Prisma Fit system (Erlangen, Germany) using a 64-channel head coil. The CSF samples were collected by lumbar draw within 6 months of MRI scan and quantification of CSF levels of Aβ42, tau, and p-tau was performed. Standard neuropsychological (NP) testing was performed on 37 of the participants to evaluate the cognitive performance. CBF, ATT and kw maps were normalized into the MNI space, and regional analysis was performed on the whole brain and in the frontal/temporal/parietal lobe, and subcortical regions including hippocampus, parahippocampal gyrus, anterior/posterior cingulate cortex (ACC/PCC), precuneus, caudate and putamen. Separate linear regression analyses were run with regional kw, CBF or ATT values as the independent variable and either CSF values or NP scores as the dependent variable and age and gender as covariates. Participants with mean scores greater than 3 SD from the group mean were excluded from individual regression models.
Results and discussion
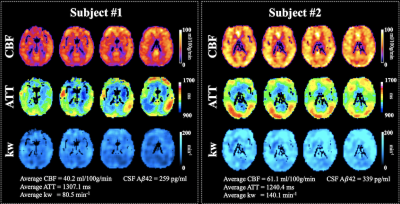
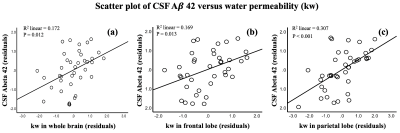
Figure 1 shows CBF, ATT and kw maps from two subject with Aβ42 = 259 and 339 pg/ml, respectively. No significant correlation was found between CBF/ATT and CSF measurements. A positive trend was found between CBF in PCC and Aβ42 (r=0.31, P=0.08).Significant associations were found between Aβ42 and kw in the whole brain (beta=0.43, t=3.2, P=0.012), frontal lobe (beta=0.43, t=2.7, P=0.013) and parietal lobe (beta=0.56, t=3.9, P < 0.001), after controlling for age and sex, as shown in figure 2. The associations between kw and Aβ42 were also significant in the precuneus (beta=0.46, t=3.1, P= 0.004) and temporal lobe (beta=0.42, t=2.6, P = 0.015). No significant association was found between kw and CSF tau or p-tau levels.
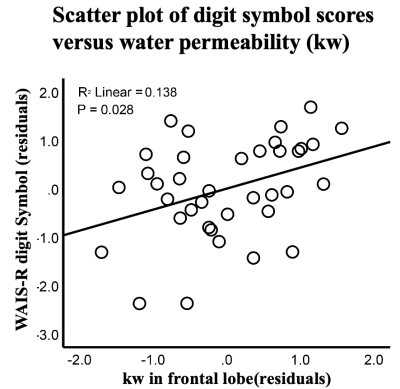
Significant positive correlations were found between digit symbol substitution test (DSST) and kw in frontal lobe (beta=0.38, t=2.3, P=0.028), as shown in figure 3. Poor performance on the DSST has been associated with neurocognitive deficits of the frontal lobe.6 The finding that subjects with lower kw have lower CSF Aβ42 levels and lower digit symbol scores indicates that altered AQP4 expression may hamper the clearance of the interstitial Aβ42, which may render the aging brain vulnerable to future neurodegenerative conditions.
Conclusion
A diffusion prepared 3D pCASL technique was proposed to measure BBB water permeability non-invasively. The significant associations between kw and CSF Aβ42 level and digit symbol scores in healthy older adult brains suggests kw may serve as an early imaging marker of AD.Acknowledgements
This work was supported by National Institute of Health (NIH) grants UH3-NS100614, R01AG055449 and P30AG028383.References
[1] Tapiola T, Alafuzoff I, Herukka S K, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain[J]. Archives of neurology, 2009, 66(3): 382-389.
[2] Zeppenfeld D M, Simon M, Haswell J D, et al. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains[J]. JAMA neurology, 2017, 74(1): 91-99.
[3] Mader S, Brimberg L. Aquaporin-4 water channel in the brain and its implication for health and disease[J]. Cells, 2019, 8(2): 90.
[4] Shao X, Ma S J, Casey M, et al. Mapping water exchange across the blood–brain barrier using 3D diffusion‐prepared arterial spin labeled perfusion MRI[J]. Magnetic resonance in medicine, 2019, 81(5): 3065-3079.
[5] St Lawrence K S, Owen D, Wang D J J. A two-stage approach for measuring vascular water exchange and arterial transit time by diffusion‐weighted perfusion MRI[J]. Magnetic resonance in medicine, 2012, 67(5): 1275-1284.
[6] Nakahachi T, Ishii R, Iwase M, et al. Frontal activity during the digit symbol substitution test determined by multichannel near-infrared spectroscopy[J]. Neuropsychobiology, 2008, 57(4): 151-158.
Figures