0029
Retrospective Vessel Selective Perfusion Imaging with Displacement Spectrum Imaging (DiSpect) at Multiple Mixing Times1Department of Electrical Engineering, University of California, Berkeley, CA, United States, 2Institute for Medical Imaging Technology, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
Synopsis
Displacement spectrum imaging (DiSpect) performs multiple scans with increasing displacement (DENSE) encodings. It can resolve the multi-dimensional spectrum of displacements that spins exhibit over the mixing time between tagging and imaging. This work presents two innovations: 1) Imaging dynamic displacement spectra by repeatedly imaging after tagging, each image corresponding to an increased mixing time. 2) Post acquisition, it is possible to retrospectively select source vessels from the displacement maps and display only their contribution to perfusion in the imaging slice. We demonstrate feasibility in flow phantom and in-vivo brain at 3T.
Introduction
The complex dynamics of flow, diffusion and perfusion are important for the study of many biophysical functions and diagnosing disease. Various tools address these phenomena[1,2], including diffusion MRI (DWI), arterial-spin labeling (ASL) and phase contrast (PC) MRI. We previously presented a new technique, Displacement Spectrum Imaging (DiSpect)[3]. DiSpect can resolve the multi-dimensional spectrum of displacements that spins exhibit over the mixing time between tagging and imaging. Previously, the technique was demonstrated by resolving displacement spectra projections along three dimensions in flow phantom and in vivo studies. Here, we present several extensions and innovations: First, we obtain several mixing times in the same scan by repeated imaging post tagging. Second, we extend this technique to acquire a fully multi-dimensional encoded displacement spectrum for every voxel in the imaging slice. In general, DiSpect data is very high-dimensional — 3 spatial, 3 displacements and a mixing time dimension. To reduce acquisition time and computation, we limit ourselves here to a single 2D slice, 2D displacement and several mixing times, resulting in a 5D space. Third, we demonstrate richness and flexibility of this multi-mixing time data by retrospectively selecting source vessels from the displacement maps. Displaying only their contribution to perfusion in the imaging slices yields a so-called retrospective, vessel-selective “ASL”-like perfusion image [4]. We demonstrate this technique in a flow/perfusion phantom and in vivo.Methods
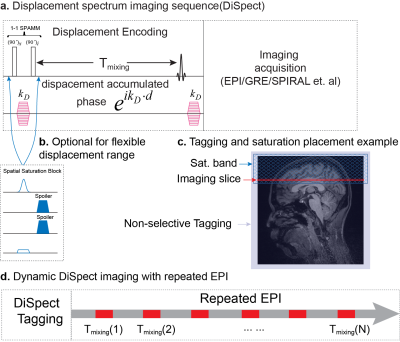
DiSpect (Fig. 1a), tags displacement using a 1-1 DENSE/SPAMM tagging pulse[5]. This is followed by an initial mixing time before an RF pulse produces the stimulated echo, which in turn is imaged using conventional imaging techniques. Here we use a single-shot EPI readout. The displacement is Fourier encoded by phase-encoding increments of the displacement encoding gradients. The displacement spectrum of each voxel is obtained by a Fourier transform along the displacement encoding dimensions. Saturation bands are applied to limit the displacement FOV and to suppress venous flow(Fig. 1b-c). To enable additional mixing times, for every tagging pulse, we perform several readouts one after the other, corresponding to higher and higher mixing times.Studies were conducted with a Siemens Trio 3T(Erlangen, Germany) scanner using DiSpect with multi-mixing-time EPI readouts. To limit scan time, a single axial image (LR and AP plane) was acquired, with displacement encoding in the orthogonal LR and SI dimensions (projection over AP). A 3D printed phantom (Figure 2) constructed to simulate vessels flowing into a bath with three compartments was imaged. A pump was used to flow water through a set of tubes and mix in a common pool full of seeds to distribute the flow. The imaging slice was placed in the pool (Figure 2b). Parameters include a spatial resolution of 3×3 mm2, 42x42 image matrix, and a displacement resolution of 3x3mm2, 40x30 displacement matrix. The measurements were taken at 100ms-750ms mixing times at 50ms intervals. In addition, an in-vivo brain scan was performed using EPI spatial resolution of 3×3 mm2, 64x64 image matrix, displacement resolution of 6×6 mm2 with a displacement FOV of 96x120 mm2, and 10 mixing-times distributed equally from 100ms to 800ms. The measurements produce a 5D dataset after coil combination consisting of the 2D imaging slice, 2D displacement spectrums and mixing times.
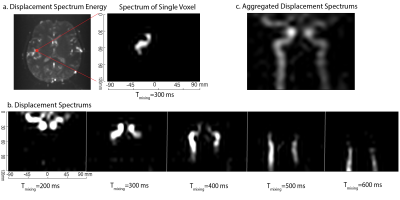
For visualization, the spectra across all voxels in the imaging slice are combined and denoised by thresholding and PCA denoising to create trace source images across different mixing times. These are expected to look similar to angiograms, as the vessels are the source feeding the slice.
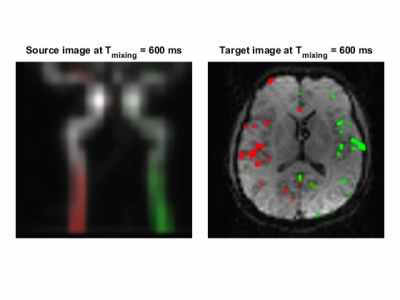
Retrospective vessel-selective perfusion imaging was performed by masking out certain regions corresponding to the tubes or vessels in the displacement spectrum and displaying their contribution within the imaged slice.
Results and Discussion
Combined trace source images for the flow phantom across 4 mixing times are shown in Figure 2c from which the shapes of the tubes can be observed. By selecting and masking out different regions of the tubes of the phantom in the displacement spectrum, the contributions of the tubes on the imaging slice can be tracked as shown in Figure 3.Figure 4 demonstrates the capability of DiSpect to resolve displacement spectrum in-vivo. By applying the same processing steps, we were able to track the blood flow through the two carotid arteries across time. We then performed the reverse analysis by masking out the two arteries and displaying their contributions in the imaging slice. The source displacement spectrums of the two arteries and their corresponding contributions in the imaging slice are displayed simultaneously across mixing time in Figure 5.
Conclusions
DiSpect with multi-mixing-time EPI readouts produces a very rich, high-dimensional dataset that can be used to resolve various complex spin dynamics. In this study, we present its ability to produce dynamic images of vessel flow as well as perform dynamic vessel-selective perfusion imaging. Although only a 2D study was conducted, DiSpect has the potential to produce a 3-dimensional displacement spectrum.Acknowledgements
We thank Kevin Johnson for extremely helpful suggestions and Ke Wang for his contributions in phantom experiments!References
1. Aletras, A. H., Ding, S., Balaban, R. S., & Wen, H. DENSE : Displacement Encoding with Stimulated Echoes in Cardiac Functional MRI, 1999, 252,: 247–252.
2. Alsop, D. C., Detre, J. A., Golay, X., et al. Recommended implementation of arterial spin-labeled Perfusion mri for clinical applications: A consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine, 2015, 73(1), 102–116
3. Zhang, Z., Karasan, E., Gopalan, K., Liu, C., Lustig, M. DiSpect: Displacement Spectrum Imaging of Flow, Diffusion and Tissue Perfusion using Spin-Labeling and Stimulated Echoes. Proc. Intl. Soc. Mag. Reson. Med. 28, Montreal, 2019.
4. Chappell, M. A., Okell, T. W., Jezzard, P., & Woolrich, M. W. A General Framework for the Analysis of Vessel Encoded Arterial Spin Labeling for Vascular Territory Mapping. Magnetic resonance in medicine, 64(5), 1529–1539.
5. Epstein, F. H., Gilson, W. D. Displacement-encoded cardiac MRI using cosine and sine modulation to eliminate (CANSEL) artifact-generating echoes. Magnetic Resonance in Medicine, 2004, 52: 774–781.
Figures