0027
Self-Regulation of Brain Functions using Real-Time Neurofeedback Functional Arterial Spin Labeling1Institute of Medical Engineering, Graz University of Technology, Graz, Austria, 2Institute of Psychology, University of Graz, Graz, Austria, 3Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 4Application Development, Siemens Healthcare, Erlangen, Germany, 5BioTechMed-Graz, Graz, Austria
Synopsis
Real-time neurofeedback (RT-NF) fMRI allows the subjects to regulate their own brain activity by providing them a neurofeedback. Functional ASL is perfectly suited for RT-NF studies due to the absolute quantification of activation related changes in the cerebral blood flow (CBF). In this study we implemented a real-time solution for ASL data processing and feedback generation which includes the following steps: data acquisition, image reconstruction, post-processing and neurofeedback presentation. The results of this RT-NF fASL study show that subjects were able to learn to regulate their own brain activation during a finger tapping experiment.
Introduction
Real-time (RT) BOLD-fMRI neurofeedback (NF) is a well-established method to regulate the brain activity of subjects by presenting them a feedback1,2. The neurofeedback allows the subjects learning to influence their own brain function which was successfully applied in patients with schizophrenia and auditory hallucinations3 or patients with chronic pain4. Functional Arterial Spin Labeling (fASL) is a very promising approach for studying the neural activation5 due to its sensitivity to blood flow alterations5 and it has some important advantages compared to BOLD fMRI which makes fASL perfectly suited for NF studies: increased spatial accuracy6, higher intra-individual reproducibility6, and a direct activation related absolute cerebral blood flow (CBF) change5. So far only one study investigated the real-time processing of fASL data7. However, that study focused only on the online monitoring of the ASL signal without providing feedback to the subject7. In this study we implemented a real-time solution for ASL data processing and feedback generation. This pipeline includes the acquisition of data, image reconstruction, post-processing and neurofeedback presentation during a finger tapping task to guide the subject’s performance. The computation of all processing steps is done within the repetition time and allows guiding the subject’s activation intermediately. The RT-NF finger tapping experiment was performed on 5 subjects. The results of this study show that the subjects were able to learn to control their own brain activation and the mean CBF in the activation area increased from 73.7±18.8 ml/100g/min without feedback to 86.2±18.9 ml/100g/min with feedback presentation.Methods
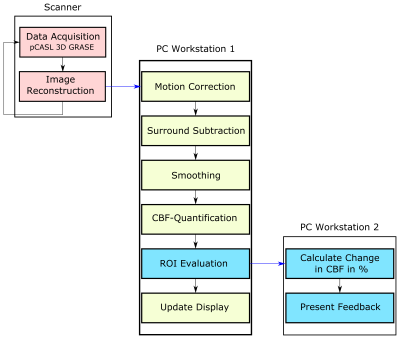
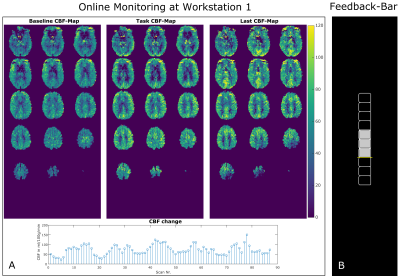
5 subjects were measured on a 3T MR system (Skyra, Siemens Healthcare, Germany) after giving written informed consent. A prototype pCASL sequence with 3D-GRASE readout was used with the following imaging parameters: FOV = 192x192x96 mm³, 3x3x6 mm3 resolution, 16 slices, phase/slice oversampling = 10/15%, slice-partial fourier (PF) = 6/8, phase-PF = 6/8, EPI-factor = 51, TF = 14, TR/TE = 4000/23ms, LD/PLD = 1800/1700ms. A baseline (BL) finger tapping experiment was conducted using a block-wise paradigm with 6 interleaved blocks (32 sec rest/task). The experiment consists of one BL run and two feedback runs (FB1 and FB2). The BL run was used to locate the activation area in the motor cortex and to create the mask for each subject.Figure 1 shows an overview of the proposed real-time neurofeedback processing pipeline. After acquisition and reconstruction on the scanner site the ASL DICOM image is transferred to workstation 1 (WS1). On WS1 the ASL preprocessing steps including motion-correction, surround subtraction8, spatial filtering, perfusion quantification9 and CBF-map visualization are performed in MATLAB (MathWorks, Natick, MA, USA) using SPM1210 and in-house MATLAB scripts. The whole procedure (from image reconstruction to presenting feedback) lasts 2s which is within the range of one TR (4s). After each acquisition the calculated CBF-maps were updated on WS1 (Figure 2) to allow monitoring the image-quality and CBF-values during the experiment. A GLM was fitted after BL scan in the CBF-time series for localization of the activation area in the motor cortex. The created mask serves as basis for FB1 and FB2. During the feedback runs the change in CBF was presented to the subject via a monitor to guide the cognitive activation. The subjects tried to modify the original finger tapping experiment which was a continuous alternating finger tapping, by changing the frequency, using different finger wiggles etc. to increase the neural activity in the motor cortex. An increase in neural activity increases the CBF and hence the feedback-bar (Figure 2B). The feedback representation was implemented in PsychoPy software.
Results and Discussion
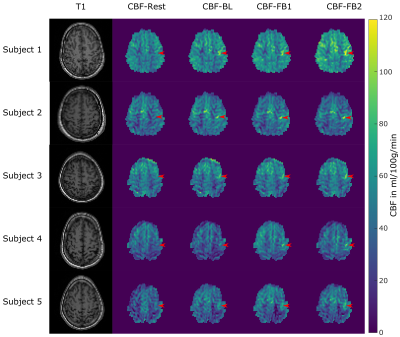
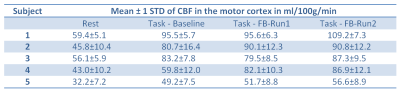
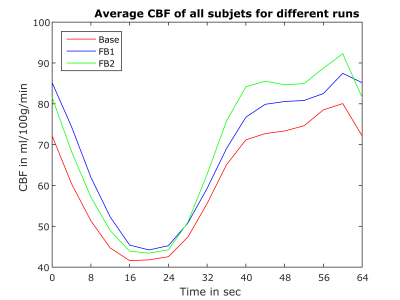
Table 1 lists the mean CBF-values in the defined activation region. Except for subject 3 we observe a slight increase in CBF of FB1 compared to BL. It should be noted that different strategies such as changing the frequency, using different finger wiggles etc. can lead to higher as well as lower CBF values in the activation area. In contrast, subject 3 was not able to find the right strategy in FB1. However, in the second feedback run (FB2) the mean CBF clearly increased for all subjects in the activation area. This indicates that the subjects were able to learn strategies to regulate their own neural activity. The CBF increase is also visible in the CBF-maps of each subject shown in Figure 3 (red arrows). Figure 4 shows the mean CBF time course of all subjects averaged over the 6 blocks. The same increase in CBF as for the individual subjects is observable with the highest increase during FB2.Conclusion
We proposed a real-time solution for fASL RT-NF studies where all necessary acquisition and processing steps were executed within a single TR. This allows to monitor the ASL signal and to guide the subjects’ cognitive process. The results of this study demonstrate that subjects can learn to regulate their brain activity during a finger tapping experiment based on the provided neurofeedback. This can be used to promote rehabilitation of different symptoms e.g. in stroke patients were motor and cognitive processes are often impaired. However only a few subjects were included in this proof of principle study and an evaluation on more subjects will be part of future work.Acknowledgements
NVIDIA Corporation Hardware grant support.References
1. Sitaram R, Ros T, Stoeckel L, et al. Closed-loop brain training: the science of NF. Nature Reviews Neuroscience 2017:18(2):86.
2. Weiskopf N, Veit R, Erb M, et al. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage. 2003:19(3):577-586.
3. Orlov ND, Giampietro V, O'Daly O, et al. Real-time fMRI neurofeedback to down-regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: a proof-of-concept study. Transl Psychiatry. 2018;8(1):46.
4. deCharms RC, Maeda F, Glover GH, et al. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102(51):18626–18631.
5. Detre JA, Leigh JS, Williams DS, et al. Perfusion imaging. Magn Reson Med. 1992;23(1):37-45.
6. Raoult H, Petr J, Bannier E, et al. Arterial spin labeling for motor activation mapping at 3T with a 32-channel coil: reproducibility and spatial accuracy in comparison with BOLD fMRI. Neuroimage. 2011;58(1):157-67.
7. Hernandez-Garcia L, Jahanian H, Greenwald MK, et al. Real-time functional MRI using pseudo-continuous arterial spin labeling. Magn Reson Med 2011;65:1570–1577.
8. Lu H, Donahue MJ and van Zijl PC. Detrimental effects of BOLD signal in arterial spin labeling fMRI at high field strength. Magn Reson Med 2006;56(3):546-52.
9. Buxton RB, Frank LR, Wong EC, et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 1998;40(3):383–396.
10. Friston KJ, Ashburner J, Kiebel S, et al. Statistical parametric mapping: the analysis of funtional brain images. Elsevier/Academic Press 2007.
Figures