0026
Towards patient specific dispersion correction for more accurate quantification in pCASL: modeling and experimental findings1Fraunhofer MEVIS, Bremen, Germany, 2MR-Imaging and Spectroscopy, Faculty 01 (Physics, Electrical Engineering), University Bremen, Bremen, Germany
Synopsis
This abstract compares quantified perfusion values of the standard model and a new dispersion model based on an AIF using the ASLIF-sequence. For different ATTs, the voxel´s signal was simulated using the dispersion model. The simulations show that the standard model overestimates the signal. This may result from lack of dispersion effects especially in the inflow phase of the labeled bolus. Consequently, the determined perfusion values vary for different ATTS. Thus, using an AIF based on an acquired patient specific reference bolus instead could improve the stability and robustness of quantified perfusion values.
Introduction
Getting reliable perfusion quantification results is one of the biggest challenges in Arterial Spin Labeling (ASL). Hereby, the underlying assumption about the shape of the Arterial Input Function (AIF) of the labeled blood bolus is crucial for robustness and stability. The boxcar shaped AIF generally used in the standard kinetic model1 is a well-known over-simplification. In fact, often the label bolus is not exactly rectangular, sometimes imperfect and most importantly, disperses during inflow. To establish ASL in clinical applications it is essential to encounter these effects in any quantification model to improve the reliability of quantified perfusion values. By utilizing the ASLIF-sequence2, label boli at two different positions were measured and were used to predict dispersion at a later arterial transit time (ATT). This will ultimately allow for the estimation of the AIF at voxel level.Methods
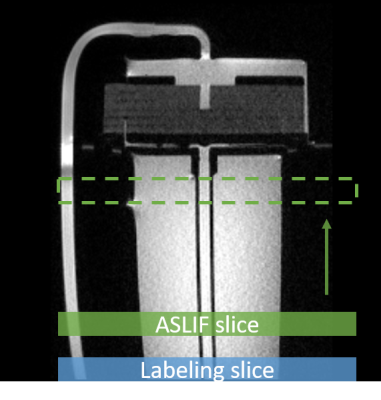
Imaging:The perfusion phantom QASPER3 was examined with an in-house developed ASLIF sequence2,4,5 on a 3T MR scanner (MAGNETOM Skyra, SIEMENS Healthineers AG). The ASLIF images were acquired with the following parameters: 4 Hadamard encoding cylces, subbolus duration (SBD) = 1400ms, post labeling delay (PLD)=1000ms, TR=6.5s, TRpCASL=1420us, phase shift = π/2 -23* π/2. The phantom had a flow rate of 350ml/min. The AIF within the main inflow vessel was determined from 12 different positions resulting in 12 different delay times (see Fig. 1). In addition, a so-called reference bolus was measured directly behind the labeling slice to yield the basis for the dispersion model.
Simulation:
A model is developed which simulates the ATT dependent shape of the label bolus during the inflow. Using this model, the signal in a voxel was simulated for ATTs between 500ms and 1600ms. The simulated measurements at three inflow times (TI) (2000ms, 3000ms and 4000ms) were then used to determine the quantified perfusion values with the standard model (MatLab, MathWorks, Natick, MA, USA). For the calculation it is assumed that the ATT is known and only the perfusion f needs to be determined. The following simulation parameters were used: f=60 ml/100g/min, T1_b=1500ms, T1_tis=2000ms, lambda=90 ml/100g, M0=500, ATT=500-1600ms, BD=1400ms, alpha=0.9.
Results
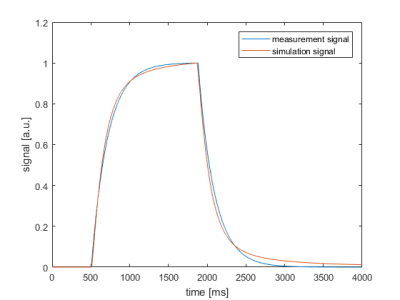
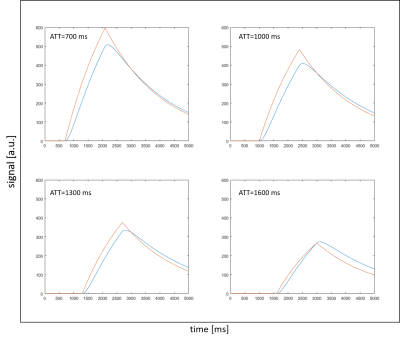
In Figure 2 the result of the developed dispersion model compared to the measurement signal is presented. A nearly identical reproducibility of the measurement can be found.The table in Figure 3a shows the fitted quantified perfusion values of the standard model for different ATTs. The determined perfusion values in the range of ATT=700-1300ms are initially overestimated with the standard model but approach the adjusted value of the dispersion model with increasing ATT (Fig. 3b). The observed relative standard deviation is in the range of 12% with a mean value of 6% over all ATTs. Figure 4 presents the fitted signal of the standard model with the quantified perfusion values resulting from the standard model fit compared to the simulated signal of the dispersion model. In all four cases, the comparison shows that the signal is overestimated during the arrival phase of the labeled bolus in the standard model compared to the dispersion model.
Discussion & Conclusion
The dispersion effects of the label bolus are not considered in the standard model. This could explain the relative error of 6 % in the quantified perfusion values. Furthermore, the phase in which the labeled bolus arrives in a voxel cannot be reproduced very well with the standard model (Fig. 4).For ATT=1300ms the signal of the dispersion model and the standard model have nearly identical parameters. However, at this point the signal of the inflow phase of the standard model is overestimated. This shows that quantification errors may occur by using the standard model. An AIF based on an acquired reference bolus can take dispersion effects into account instead of a boxcar shaped AIF.
The comparison of the two models shows how important it is to consider dispersion effects for a robust quantification.
Using the ASLIF sequence, it is possible to measure a reference bolus directly behind the labeling slice. This reference bolus represents the individual resulting AIF of the labeling. By using this reference bolus with the dispersion model instead of a boxcar shaped AIF, it can be possible to detect and extrapolate dispersion effects, disturbances or deviations in the labeling process. The incorporation of a reference bolus for an ATT dependent AIF could improve the robustness and stability of perfusion quantification and may therefore be an important step towards the clinical application of ASL.
Acknowledgements
References
1. Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelmann RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magnetic resonance in medicine. 1998;40(3):383-396.
2. Guenther M. Arterial Spin Labeled Input Function (ASLIF): signal acquisition during pseudo-continuous arterial spin labeling in Proceeding of the 26th Annual Meeting of ISMRM; 2018; Paris, France.
3. Oliver-Taylor A, Goncalves M, Hampshire T, Davis B, Daga P, Evans L, Bainbridge A, Wheeler-Kingshott C, Sokolska M, Thornten J, Vita ED, Golay X. A Calibrated Perfusion Phantom for Quality Assurance of Quantitative Arterial Spin Labelling in Proceeding of the 25th Annual Meeting of ISMRM; 2017; Honolulu, USA.
4. Günther M, Highly efficient accelerated acquisition of perfusion inflow series by cycled arterial spin labeling in Proceeding of the 15th Annual Meeting of ISMRM; 2007; Berlin, Germany.
5. von Samson-Himmelstjerna F, Madai VI, Sobesky J, Guenther M. Walsh-ordered hadamard time-encoded pseudocontinuous ASL (WH pCASL). Magnetic resonance in medicine. 2016;76(6):1814-24.
Figures