0023
High-resolution whole brain ASL perfusion imaging at 7T with 12-fold acceleration and spatial-temporal regularized reconstruction1Mark & Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Institute of Medical Engineering, Graz University of Technology, Graz, Austria, 3Department of Neurology, University of Southern California, Los Angeles, CA, United States
Synopsis
Ultra-high field allows ASL to achieve higher spatial resolution due to increased SNR and prolonged T1 relaxation. We present a single-shot 3D GRASE pCASL technique with 12-fold acceleration using time-dependent 2D CAIPI sampling strategy, and reconstruction of the label/control time series with joint spatial and temporal total-generalized-variation (TGV) regularization. 2D CAIPI under-sampling pattern increases temporal incoherence between measurements which allows joint reconstruction of the highly accelerated ASL time series. Combining the advantages of ultra-high field strength, pTx coils, accelerated acquisition and advanced reconstruction, whole-brain CBF map with 2 mm isotropic resolution was obtained within 5 mins.
Introduction
Pseudo-continuous arterial spin labeling (pCASL) with segmented 3D acquisitions has been recommended for clinical implementations with moderate spatial resolution (>3 mm) at 3T due to the low SNR.1 Ultra-high field allows ASL to achieve higher spatial resolution due to increased SNR and prolonged T1 relaxation.2,3 However, standard segmented 3D acquisition is not ideal for higher resolution due to increased motion sensitivity and image blurring effect. In this study, we present a motion-robust single-shot 3D GRASE pCASL technique at 7T with a 12-fold acceleration using time-dependent 2D CAIPI sampling strategy to increase the temporal incoherence.4 To exploit this, the whole label/control time series were jointly reconstructed using spatio-temporal total-generalized-variation (TGV) regularization.4 The combination of ultra-high field strength, parallel RF transmit (pTx) coils, accelerated acquisition and advanced reconstruction yields high resolution (2 mm isotropic) whole brain cerebral blood flow (CBF) maps within an acquisition time of 5 mins.Methods
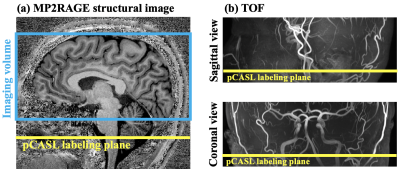
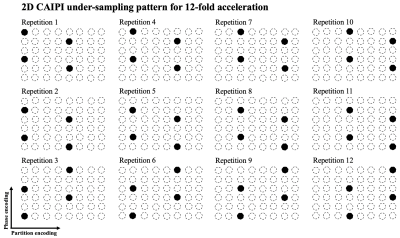
Two healthy subjects (2M, age=31.0±5.0 yrs) underwent MRI scan on a 7T Siemens Terra scanner with a NOVA 8-channel parallel transmit (8-pTX) and 32-channel receiver (Rx) head coil. A standard and an accelerated ASL acquisition were performed with the following common imaging parameters: FOV=200 mm, matrix size=96×96, 40 slices with 20% slice oversampling, resolution = 2.1×2.1×2 mm3. pCASL parameters were: FA=250, Gmean = 0.6 mT/m, Gratio = 10, labeling duration = 1500 ms and post-labeling delay = 1800 ms. Position of imaging volume is shown in figure 1 (a). pCASL labeling plane was placed to be approximately perpendicular to both carotid and vertebral artery based on both structural (figure 1 (a)) and TOF MRA images (figure 1 (b)). Labeling efficiency was derived by Bloch equation simulations assuming flow velocity = 40 cm/s at carotid artery. Background suppression (BS) was applied to suppress gray and white matter signals. SAR was monitored by FDA approved vendor software and was within the first level (3.2W/kg on head).For the standard acquisition with segmented readouts: turbo factor = 12, EPI-factor = 31, 12 segments, TE = 22 ms and TR = 6000 ms. One M0 and one pair of label/control images were acquired in 3 mins 36 secs. Single-shot 3D-GRASE acquisition was achieved with 12-fold acceleration using a time-dependent 2D-CAIPI under-sampling pattern to increase the temporal incoherence between measurements, as illustrated in Figure 2. Coil sensitivity maps were estimated using ESPIRiT5 from combined k-space of 12 measurements. The whole 4D under-sampled label/control time series were jointly reconstructed using spatial-temporal TGV regularized reconstruction.4 For the accelerated acquisition, two M0-images and 24 pairs of label/control images were acquired in 5 mins. Motion correction was performed using SPM126 and ASL-Toolbox.7,8 CBF maps were calculated according to ASL white paper.1
Results and discussion
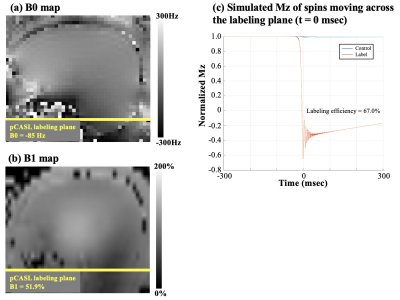
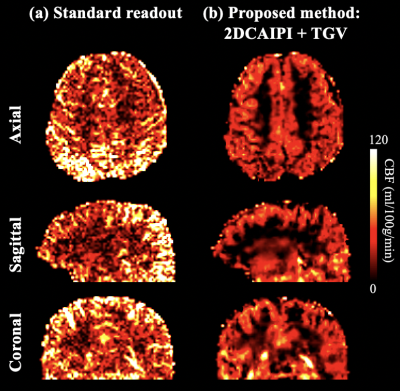
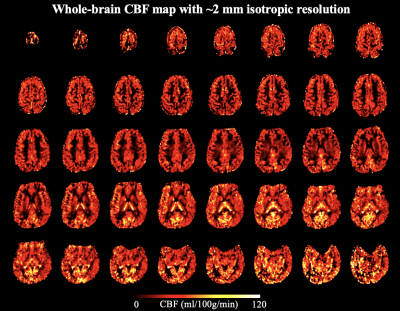
Average B0 = -85 Hz and average B1 = 51.9% with True-Form B1-shimming at the pCASL labeling plane, as shown in Figure 3 (a) and (b). Figure 3 (c) shows simulated magnetization of spins flowing through the labeling plane. Labeling efficiency = 67%, which is lower than typical pCASL at 3T mainly due to B1 insufficiency. B1-shimming for pCASL labeling could be improved using advanced pTx configuration for the next step.Figure 4 (a) shows CBF maps calculated from standard fully sampled acquisition in axial, sagittal and coronal views. The high number of segments (tacq = 1 min 12 secs) of the standard acquisition makes the ASL signal very sensitive to motion and physiological fluctuations especially when spatial resolution is high. Figure 4 (b) shows CBF maps calculated from accelerated acquisition with spatial-temporal TGV reconstruction. With high spatial resolution and sufficient SNR, perfusion along cortical gyri can be observed. 2D CAIPI under-sampling pattern increases temporal incoherence between measurements which allows joint reconstruction of the highly accelerated label/control time series. Figure 5 shows forty axial slices of CBF maps acquired with 2D CAIPI readout. The proposed technique allows whole brain coverage with 2 mm isotropic resolution in 5 mins at 7T.
Conclusion
Standard segmented 3D ASL suffers from motion and physiological fluctuations during the long acquisition window especially for high spatial resolution. To overcome this problem we proposed a single-shot 3D GRASE pCASL sequence with 12-fold acceleration and time-dependent 2D CAIPI pattern. The temporal incoherence of the sampling is directly exploited in the reconstruction approach and additional spatial and temporal constraints on the individual images improved the SNR. By combining the advantages of ultra-high field strength, pTx coils, accelerated acquisition and advanced reconstruction, whole-brain CBF map with 2 mm isotropic resolution obtained within 5 mins.Acknowledgements
This work was supported by National Institute of Health (NIH) grant UH3-NS100614 and R01-EB028297. NVIDIA Corporation Hardware grant support.References
[1] Alsop D C, Detre J A, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia[J]. Magnetic resonance in medicine, 2015, 73(1): 102-116.
[2] Zuo Z, Wang R, Zhuo Y, et al. Turbo-FLASH based arterial spin labeled perfusion MRI at 7 T [J]. PloS one, 2013, 8(6): e66612.
[3] Pohmann R, Speck O, Scheffler K. Signal‐to‐noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays[J]. Magnetic resonance in medicine, 2016, 75(2): 801-809.
[4] Spann S M, Shao X, Wang DJJ, et al. Robust single-shot acquisition of high resolution whole brain ASL images by combining time-dependent 2D CAPIRINHA sampling with spatio-temporal TGV reconstruction [J]. Neuroimage, 2019 (accepted).
[5] Uecker M, Lai P, Murphy M J, et al. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA[J]. Magnetic resonance in medicine, 2014, 71(3): 990-1001.
[6]. Friston KJ, Ashburner J, Kiebel S, et al. Statistical parametric mapping: the analysis of funtional brain images. Elsevier/Academic Press 2007.
[7] Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn. Reson. Imaging 2008;26:261–269.
[8] Wang Z. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn. Reson. Imaging 2012;30:1409–1415.
Figures