0016
Human cerebral white-matter vasculature imaged using the blood-pool contrast agent Ferumoxytol: bundle-specific vessels and vascular density
Michaël Bernier1,2, Olivia Viessmann1,2, Ned Ohringer1, Jingyuan E. Chen1,2, Nina E. Fultz1,3, Rebecca Karp Leaf4, Lawrence L. Wald1,2,5, and Jonathan R. Polimeni1,2,5
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Radiology, Harvard Medical School, Boston, MA, United States, 3Engineering, Boston University, Boston, MA, United States, 4Division of Hematology, Massachusetts General Hospital, Boston, MA, United States, 55Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Radiology, Harvard Medical School, Boston, MA, United States, 3Engineering, Boston University, Boston, MA, United States, 4Division of Hematology, Massachusetts General Hospital, Boston, MA, United States, 55Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Ferumoxytol—a safe, superparamagnetic iron oxide nanoparticle that amplifies T2* dephasing in blood vessels—can be used as a powerful image contrast enhancement agent to aid vascular imaging. Combining this with an innovative vascular segmentation tool, here we evaluate how Ferumoxytol improves vascular detection throughout the brain using a region-based analysis of the gray-matter and a bundle-specific analysis of the white-matter. We report increases in white-matter vasculature specificity and uncover spatial patterns similar to white-matter tracts, therefore this work sheds new light on the possible existence and influence of a concurrent network of vasculature that follows the known fiber bundles.
INTRODUCTION
Cerebral vascular imaging provides crucial information in clinical research to study vascular impairment in healthy and diseased brain function, but also in neuroscientific research to assess vascular biases in fMRI methods [1]. However, human in vivo imaging technologies are currently limited in terms of their capacity to measure the smaller vessels of the cerebrovasculature (< 200 μm) [2], amongst them white-matter vessels, and thus our understanding of the pathophysiology of the many white-matter diseases with vascular underpinnings is limited. To overcome this, Ferumoxytol, a safe iron supplement administered intravenously [3], can be used as an MR blood-pool contrast agent to improve detection of white matter vessels. It provides a long (~12-hour) half-life, strong T1 and T2* shortening, and no extravasation into surrounding tissues [4], [5]. Ferumoxytol could therefore help investigate the cortical, subcortical, and deep white matter (WM) arteries or deep medullary veins usually observed post-mortem or at high field MRI [6]–[8], allowing for an improved detection of the brain vasculature throughout all tissue types or for a better measurement of vascular defect in the deep WM vessels [9]. Given this, our main objectives were to (1) explore the vascular detection and segmentation improvements provided by Ferumoxytol, in order to (2) find small vessels normally undetected in all tissues including the WM without contrast agent and, (3) quantify the vascular densities across different gray-matter (GM) regions and WM fiber-bundles.METHODS
Using a Siemens TimTrio 3T scanner, pre- and post-injection with 510 mg of Ferumoxytol, four anemic but neurologically healthy (44 ± 7 y.o., 3F) underwent MRI sessions (one day apart) consisting of an anatomical T1-weighted MP2RAGE acquisition (TR/TI1/TI2/TE=5000/700/2500/2.5 ms, voxel size 1 mm³ iso.), a 15-minute whole-head 3D T2* multi-echo GRE acquisition (192×192×96 mm FOV, 7 echoes, TR/TEs=2000/4.88/9.76/14.64/19.52/24.40/29.28/34.16 ms, voxel size 0.6 mm³ iso., flip angle=17°). All pre- and post-injection images were non-linearly coregistered symmetrically to their mid-point (T2*-mid and T1-mid). The T2*-mid pre- and post-images were then non-linearly coregistered to corresponding T1-mid pre- and post-images using ANTs [10]. The T1-mid pre anatomical image was segmented using FreeSurfer and non-linearly registered to the MNI space to allow a back-projection of the IIT 5.0 WM bundle atlas (obtained from RecoBundle [11], [12]) each subject. This resulted in 82 GM regions and 24 WM bundles (contiguous overlapping 3D masks representing specific major fiber bundles) in subject space. All ME-GRE echoes were individually denoised using non-local means (NLM), N4 bias-corrected and skull-stripped using ANTs. Vascular segmentation was performed on all echoes using an updated Braincharter segmentation tool [13], limited to a diameter range between 0.5 and 3.0 mm, which generated a "vesselness" score that was then thresholded at >95% to obtain a fine vessel tree. GM vessels were identified as those above the WM surface, and WM vessels were identified as those within a bundle ROI.RESULTS
Figure 1 illustrates the segmentation results for a single subject; Ferumoxytol allowed an enhanced depiction of the vasculature, especially within the WM. Figure 2 shows the regional vascular density for both the GM and WM, calculated at the group level, with and without the contrast agent. In the WM, an increase (an average of 3.76 times more) was observed for all bundles tested. Even though our sample size is limited, the vascular density was consistent amongst the subjects. Figure 3 shows the spatial patterns of the vasculature from a small subset of selected WM bundles with the highest density.DISCUSSION & CONCLUSION
Although the vascular density varied differently from region to region with Ferumoxytol, the global spatial pattern of the GM vascular distribution agrees with the pattern without Ferumoxytol and with our previous study [13]. Several WM bundles exhibited a higher vascular density than others. Surprisingly, the fornix was found highly vascularized, which could be caused by its relatively smaller size compared to the other bundles. In several bundles the spatial pattern of the vessels qualitatively appears to follow the coarse-scale geometry of the fibers. While this requires validation, if true this finding may help inform our interpretation of the geometry of fiber bundles estimated from diffusion MRI—since luminal signal from slow blood flow may impact the apparent diffusion coefficient at low b-values, and vessel walls themselves might hinder and restrict tissue water diffusion for higher b-values [14]. A limitation of our study is that these data cannot distinguish between a bundle having larger vessels compared to many sub-voxel vessels. Nevertheless, our white matter atlas, the first created from in vivo data, could provide valuable information regarding the anatomical relationships between the white matter vasculature and fiber bundles, especially as it is possible to exhibit extensive vascular injury without adjacent axonal injury [15].Acknowledgements
This work was supported in part by the NIH NIBIB (grants P41-EB015896 and R01-EB019437), NINDS (grant R21-NS106706), by the BRAIN Initiative (NIH NIMH grant R01-MH111419), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grants S10-RR023043 and S10-RR019371. We thank our colleagues at Siemens Heathineers for use of the Works-In-Progress package #944, and Dr. Brian Edlow for sharing his thoughts on clinical applications.References
[1] S. M. Kazan et al., “Vascular autorescaling of fMRI (VasA fMRI) improves sensitivity of population studies: A pilot study.,” Neuroimage, vol. 124, no. Pt A, pp. 794–805, Jan. 2016. [2] M. Marín-Padilla, “The human brain intracerebral microvascular system: development and structure,” Front. Neuroanat., vol. 6, p. 38, Sep. 2012. [3] K.-L. Nguyen et al., “Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI,” Radiology, p. 190477, Oct. 2019. [4] A. M. Muehe et al., “Safety Report of Ferumoxytol for Magnetic Resonance Imaging in Children and Young Adults.,” Invest. Radiol., vol. 51, no. 4, pp. 221–227, Apr. 2016. [5] E. A. Neuwelt et al., “The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: A pilot study,” Neurosurgery, vol. 60, no. 4, pp. 601–611, Apr. 2007. [6] H. Nonaka, M. Akima, T. Hatori, T. Nagayama, Z. Zhang, and F. Ihara, “Microvasculature of the human cerebral white matter: Arteries of the deep white matter,” Neuropathology, vol. 23, no. 2, pp. 111–118, 2003. [7] T. P. Naidich, H. M. Duvernoy, B. N. Delman, A. G. Sorensen, S. S. Kollias, and E. M. Haacke, Duvernoy’s Atlas of the Human Brain Stem and Cerebellum. Vienna: Springer Vienna, 2009. [8] H. J. Kuijf et al., “Quantification of deep medullary veins at 7 T brain MRI,” Eur. Radiol., vol. 26, no. 10, pp. 3412–3418, Oct. 2016. [9] Y. Hase et al., “White matter capillaries in vascular and neurodegenerative dementias,” Acta Neuropathol. Commun., vol. 7, no. 1, p. 16, Dec. 2019. [10] B. Avants, N. Tustison, and G. Song, “Advanced Normalization Tools (ANTS),” Insight J., pp. 1–35, 2009. [11] E. Garyfallidis et al., “Recognition of white matter bundles using local and global streamline-based registration and clustering,” Neuroimage, vol. 170, pp. 283–295, Apr. 2018. [12] S. Zhang and K. Arfanakis, “Evaluation of standardized and study-specific diffusion tensor imaging templates of the adult human brain: Template characteristics, spatial normalization accuracy, and detection of small inter-group FA differences,” Neuroimage, vol. 172, pp. 40–50, May 2018. [13] M. Bernier, S. C. Cunnane, and K. Whittingstall, “The morphology of the human cerebrovascular system,” Hum. Brain Mapp., vol. 39, no. 12, pp. 4962–4975, Dec. 2018. [14] P. Heusch et al., “Impact of blood flow on diffusion coefficients of the human kidney: A time-resolved ECG-triggered diffusion-tensor imaging (DTI) study at 3T,” J. Magn. Reson. Imaging, vol. 37, no. 1, pp. 233–236, Jan. 2013. [15] A. D. Griffin et al., “Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury,” Brain, pp. 1–15, Oct. 2019.Figures

Vascular segmentation before and after Ferumoxytol administration.
Pre-injection/long-TE (TE=30 ms) image to maximize the vascular/tissue contrast vs. a post-injection/short-TE (5 ms) image to minimize vessels blooming and the dephasing effect induced by the blood-pool contrast agent. An increased amount of vasculature detected across the whole brain (A) is detected after administration of the contrast agent. (B) Above the WM surface, a similar increase is observed. (C) WM vessels (noisy and disconnected) can be seen pre-contrast, whereas WM vessels increased dramatically.
Pre-injection/long-TE (TE=30 ms) image to maximize the vascular/tissue contrast vs. a post-injection/short-TE (5 ms) image to minimize vessels blooming and the dephasing effect induced by the blood-pool contrast agent. An increased amount of vasculature detected across the whole brain (A) is detected after administration of the contrast agent. (B) Above the WM surface, a similar increase is observed. (C) WM vessels (noisy and disconnected) can be seen pre-contrast, whereas WM vessels increased dramatically.
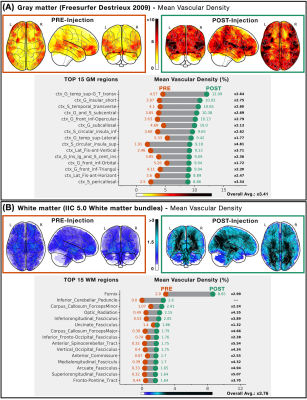
Vascular density across gray-matter regions and
white-matter bundles. The mean within-subject
vascular density is reported for (A) GM regions from Freesurfer's probabilistic atlas and (B) WM bundles from the IIC 5.0
probabilistic atlas. For both panels, Vascular densities of all regions/bundles are color-coded in a glass-brain projection (pre: orange, post: green). Bottom graph
represents the top 15 regions/bundles sorted by post-injection densities (relative pre-post increase in black; mean GM increase: x3.41, mean WM increase: x3.76).
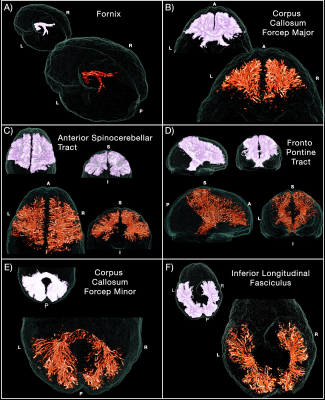
Vascular white-matter bundles. Using
IIT white-matter bundles as a 3D selection mask (shown in each panel in white),
the vascular segmentations in post-injection images were isolated and shown
here (in orange) to illustrate the directional pattern of the vasculature in
major bundles. Here we illustrate amongst the most vascularized white-matter
bundles (A) the fornix, the (B) corpus callosum (major), the (C) anterior
spinocerebellar tract, the (D) fronto-pontine tract, the (E) corpus callosum
(minor), and the (F) inferior longitudinal tract.