0015
Distribution of intraperitoneally administered D2O in AQP4-knockout mouse brain after MCA occlusion1National Institute of Radiological Sciences, QST, Chiba, Japan, 2Department of Pharmacology, Keio University School of medicine, Tokyo, Japan
Synopsis
Using dynamic PDWI after intraperitoneal D2O injection, we observed a difference in the D2O distribution between aquaporin-4 knockout (AQP4-ko) and wild type (Wild) mice with MCA occlusion. The results suggest that blood flow changes and cell membrane water permeability have a complex relationship.
Introduction
Aquaporin-4 (AQP4) is a protein complex embedded in cell membranes that facilitates the exchange of water molecules from one side of the membrane to the other. In direct measurements using optical imaging and indirect studies using diffusion MRI, different levels of AQP4 expression have been found to affect cell membrane water permeability [1]. AQP4 knockout mice (AQP4-ko) could be useful for clarifying the role of AQP4 in brain water dynamics, but it is difficult to monitor the dynamics of H2O in vivo.An imaging method based on deuterium-labeled water (D2O) may be useful for this purpose. Proton-density-weighted imaging (PDWI) can visualize proton signal from water and fat. Because the process of exchanging H2O with D2O corresponds to signal loss in PDWI, it is possible to monitor the in vivo dynamics of D2O. One problem for the administration of D2O to mice is the very small blood volume of a mouse (2-3 ml). If an intra-venous injection of 1ml D2O is performed, the circulation system would be severely affected. Therefore, we planned to administer D2O intraperitoneally so that the exchange between intraperitoneal D2O and intravascular H2O is less dramatic. In AQP4-ko mice with a brain infarction, it has been reported that the DWI signal change is different from that for wild-type mice. In this study, dynamic PDWI was performed with D20 i.p. injection to compare the distribution of D2O in wild type mouse (Wild-type) and AQP4-ko brains after middle cerebral artery (MCA) occlusion.
Methods
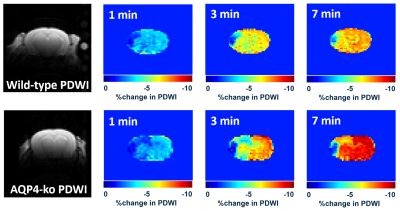
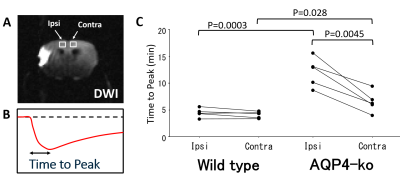
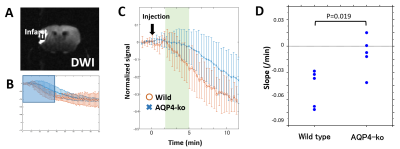
Five Wild-type and five AQP4-ko mice were used for this study. A surgical procedure (Tamura method [2]) to occlude the stem of the unilateral proximal MCA was performed 3 hours before beginning the dynamic PDWI scans. Dynamic PDWI was performed with a 7T animal MRI (Bruker BioSpin70/20USR, 4-shot spin-echo EPI, TR = 5s, TE = 11ms, FOV = 19.2mm, 64x64 matrix, 1mm thickness) by acquiring an image every 20 s for 30 minutes (total 90 scans). Two minutes after the scan start, 1 ml of D2O saline was intraperitoneally administered. In addition, DWI (b=1000 s/mm2) was taken to confirm ischemic changes.In order to evaluate PDWI signal changes after D2O administration, ROIs were drawn at the following three locations: DWI high signal region in the MCA area (INFARCT), the cortex in the ACA territory on the same side (Ipsi-ACA), and the cortex in the opposite ACA territory (Contra-ACA). For the Contra-ACA and Ipsi-ACA ROIs the signal changes after D2O administration were fitted to polynomial curves, and the time from D2O administration to the minimum signal value was estimated as Tpeak. For the INFARCT ROI, a signal minimum was never reached for 8 of the 10 subjects so the slope of the time-intensity curve (3-5min) was estimated as an alternative parameter (Slope) to characterize signal change.
Results and Discussion
In the DWI performed prior to dynamic PDWI, all mice showed a high signal region in the ipsilateral MCA territory. Signal decreases in the brain were observed for all mice immediately after intraperitoneal administration (Fig. 1), which is considered to reflect the transfer of D2O into the brain. In the ipsilateral MCA territory, there was a region where the signal drop was delayed. For the Wild-type, signal changes in the Ipsi-ACA ROI were similar to those observed in the Contra-ACA ROI, whereas the signal changes looked quite different for the AQP4-ko (Fig. 2).There was no difference in Tpeak between the Contra-ACA and Ipsi-ACA for the Wild-type, while for the AQP4-ko a significant difference was observed (Fig. 3). There were also significant differences in the Contra-ACA and Ipsi-ACA estimates of Tpeak between the two mouse types, although the significance quite weak (p=0.028) for the Contra-ACA ROI. In general, when ligation is performed at the main trunk of the MCA, arterial blood is partially compensated by flow from the ACA, which means that supply to the ACA region is reduced. The elongation of Tpeak for the Ipsi-ACA ROI of AQP4-ko mice seems to be naturally affected by this CBF reduction, while the fact that there was no significant change for the Wild-type mice may need explanation. One possibility is that the number of active AQP4 sites increases in the Ipsi-ACA region of the Wild-type mice so that the water transfer to the brain is maintained even under the condition of low CBF.
As for the INFARCT ROI, the Slope for Wild-type mice (-0.016 ± 0.0070/min) was smaller than that for AQP4-ko mice (-0.0030 ± 0.0069/min), and there was a significant difference between them (Fig. 4). The infarct region is thought to have almost no blood flow for both Wild-type and AQP4-ko due to the MCA occlusion. The difference in the INFARCT signal change may be due to the faster inflow of D2O from the surrounding intact area in the Wild-type mice.
In conclusion, dynamic PDWI with D2O i.p. is useful for imaging D2O dynamics in mouse brain. Under severe ischemic conditions, the difference between the Wild and AQP4-ko mice was significant. This may be because there is some compensation mechanism for the maintenance of intracerebral water transfer, and this fails for the AQP4-ko mice in a pathological state.
Acknowledgements
This research was supported by a Grant-in-Aid for Scientific Research (Kakenhi #15H04910) from the Japan Society for the Promotion of Science (JSPS), by AMED under Grant Number 16cm0106202h and 17dm0107066h, and by a grant (COI stream) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japanese Government.References
1. Obata, T., et al., Comparison of diffusion-weighted MRI and anti-Stokes Raman scattering (CARS) measurements of the inter-compartmental exchange-time of water in expression-controlled aquaporin-4 cells. Sci Rep, 2018. 8(1): p. 17954.
2. Tamura, A., et al., Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab, 1981. 1(1): p. 53-60.
Figures