Mechanisms of Brain Drain: Glymphatics
1Anesthesiology, Yale University, New Haven, CT, United States
Synopsis
The glymphatic system (GS) is described as a perivascular transit passageway for cerebrospinal fluid (CSF) and interstitial fluid exchange that facilitate metabolic waste drainage from the brain in a manner dependent on aquaporin 4 (AQP4) water channels on glial cells and vascular pulsatility. In this presentation I will present the fundamentals of the GS concept (including current controversies) and focus on MRI methods to quantify GS transport in normal and diseased brain. Evidence for its existence in human brain will also be highlighed.
Target audience
Imaging scientists/radiologists, physiologists, neuroscientistsLearning objectives
- The exact processes involved in cerebrospinal fluid (CSF) facilitated waste clearance from brain remain poorly understood. This presentation will present recent information pertaining to the ‘glymphatic system’, a peri-vascular pathway dedicated to metabolic waste drainage from the brain. Controversies in the literature will be highlighted.
- We will discuss MRI methods developed to quantify glymphatic transport in the brain using MRI contrast agents administered into CSF as surrogate small molecular weight waste solutes.
- This presentation will discuss pros and cons of the different approaches to quantify CSF transport via the glymphatic system from the MRI based techniques and highlight quantitative features of relevance to neurodegeneration.
- Examples from preclinical studies in rodents as well as more recent studies in humans will be presented
Purpose: Understanding brain waste drainage and the glymphatic system concept
The brain’s unremitting high energy demand is paralleled by metabolic waste production at a higher rate than in other organs. In most body organs the lymphatic vasculature is responsible for metabolic waste drainage and fluid homeostasis. While the meninges covering the brain and spinal cord are equipped with lymphatics 1-3, the brain parenchyma itself is devoid of authentic lymphatic vessels. The presence of tight blood brain barrier (BBB) restricts solute and large fluid shifts and alternate waste elimination systems are operational in brain tissue. CSF production and transport has been studied for decades from the point of view of facilitating metabolic waste drainage from the brain parenchyma. The “glymphatic system” concept was introduced in 20124 and a multitude of novel studies have since emerged shedding new light on CSF transport and brain waste drainage processes in animals and humans. The glymphatic system (GS) is a perivascular transit passageway for CSF and interstitial fluid (ISF) exchange that facilitate metabolic waste drainage from the brain parenchyma in a manner dependent on aquaporin 4 (AQP4) water channels on glial cell4. Most importantly, CSF transport via the GS system appears to be almost non-functional in the awake state and waste drainage is most efficient during sleep and anesthesia5 (although not all anesthetics have the same effects on transport efficiency6,7).
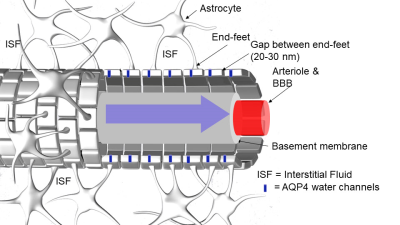
The GS is located beyond the BBB and comprises the entire peri-vascular space (PVS) of the brain4. The PVS is constructed as a coaxial system where the inner cylinder is the BBB-tight vessel (e.g. artery, arteriole, capillary, venule or vein) and the outer cylinder is made of astrocytic end-feet processes which envelop the entire cerebral vasculature (Fig. 1). The astrocyte end-feet perimeter of the PVS is not ‘tight’ as the BBB due to gaps (20-30 nm) between the end-feet processes8. In humans, PVS can be detected in the brain parenchyma even in young healthy subjects by MRI and run perpendicular to the brain’s surface in directions that are spatially correlated with perforating vessels thought to be arterial9. In the human and rodent brain, the PVS communicates with the subarachnoid space as evidenced by multiple studies showing that tracer uptake is visible in PVS following in vivo administration of tracers into the CSF via the cisterna magna. The importance of the AQP4 water channels for rapid CSF-ISF exchange has been contentious10-12, and alternate physiological factors (e.g. ISF volume, vascular and respiratory pulsatility) may be more important for time efficient GS transport and waste drainage. It is also not known exactly how the AQP4 water channels regulate GS function. However, decreased peri-vascular AQP4 expression is associated with declining GS function and Alzheimer's disease in human post-mortem tissue.
MRI methods for tracking whole brain GS transport
To visualize and quantify GS function in the whole rodent brain we developed a MRI based method by administered paramagnetic gadolinium-tagged contrast molecules into CSF of the rat via the cisterna magna in combination with dynamic contrast enhanced (DCE) T1-weigthed MRI13. We demonstrated that small MW paramagnetic contrast molecules moved rapidly in the subarachnoid space, along pial arteries and more slowly transited into brain parenchyma in a specific anatomical pattern13. We noted that brain regions with most rapid CSF and solute transport included the brainstem, hypothalamus, olfactory bulb, frontal cortex, cerebellum and the ventral hippocampus13. The GS transport pattern in the rat brain using DCE MRI and MR contrast is very similar to that observed using the same method in non-human primate brain14 and humans15-17. We have since refined the MRI based GS transport technique and determined than in the rat brain only a relatively small (19-20%) fraction of the contrast agent administered into the CSF enters into the brain parenchyma over 2.5 hrs18. In addition, we showed that MR contrast influx and clearance from brain parenchyma is dependent on body position19 and the anesthetic used20. Other recent MRI efforts to measure peri-vascular CSF transport implemented ultra-long echo time (TE), diffusion weighted MRI sequences to assess fluid movement within perivascular channels on the surface of the brain at the level of the circle of Willis including middle cerebral artery (MCA) of the healthy rat brain; and showed that fluid movement were directional and dependent on cardiac pulsations21. So far, this method has not been applied to study CSF transport along the PVS in brain parenchyma.Quantification of GS transport
Current techniques for quantifying GS transport based on DCE MRI include, assessment of time-signal-curves of brain parenchymal solute uptake or clearance13,16,20, kinetic analysis19 or k-means cluster analysis13,22. All of these analytical strategies are useful and have provided valuable information but are limited; for example, kinetic and cluster analysis strategies only provide a static ‘snapshot’ of GS transport over 2-3 hrs and dynamic information is lost. Furthermore, kinetic modeling of whole brain GS transport can be problematic because GS transport kinetics varies across brain regions. We were the first to model glymphatic system transport based on DCE MRI images using the traditional optimal mass transport (OMT) formulation 23,24. The theory of OMT seeks the most feasible way to redistribute mass from one given distribution to another while minimizing the associated cost of transportation25. Initially, we made several assumptions including the notion that glymphatic CSF-solute transport was governed principally by advection23. While these initial results revealed unique CSF “streaming” patterns of MR contrast solutes into brain parenchyma, there was ‘misalignment’ with the physiological expectations of GS transport patterns based on the raw MRI contrast images23. We have now further improved the OMT based computational analysis with the ultimate goal of visualizing how PVS pathology alter fluid transport in neurodegeneration such as cerebral small vessel disease. We introduced a novel visualization framework, “GlymphVIS”26 and a more physiologically relevant model inspired by the work of Benamou and Brenier27. Specifically, in the GlymphVIS model we implemented a diffusion term in the standard continuity equation to better model both advection and diffusion in the GS thereby more accurately modelling the CSF-solute transport behavior in the tissue (for more detail see Elkin et al., 201826). Fig. 2a shows the effect of increasing the diffusion term in the algorithm and clearly shows that CSF parenchymal streamlines with minimal diffusion ‘weighting’ are aberrant and showing up in brain areas where CSF transport efficiency is poor on the original DCE MRIs. In contrast, with more diffusion ‘weighting’, (Fig. 2b) CSF streamlines derived by OMT analysis are aligning better with physiological visualization by DCE MRI in live rodent brain strongly suggesting that GS transport in the live rodent brain is governed by both advection and diffusion. We are in the process of further validating these data using phantoms with predetermined convective profiles. Moreover, we are exploring and comparing the advantages (and disadvantages) of both Eulerian and Lagrangian coordinates in visualizing the flow.Conclusion and human studies
MRI based methods for measuring GS transport in the whole brain continue to evolve, however most of the DCE MRI techniques are favoring CSF ‘influx’ pathways rather than solute clearance. New studies applying non-invasive MRI and ultrasound modalities are underway which will specifically target visualization and quantification of solute clearance from brain parenchyma to the outside authentic lymphatics. As of now, no single study has unequivocally affirmed the existence of the GS in the human brain, however the confluence of findings from CSF and brain imaging studies provide indirect evidence of its presence. Recently, human subjects undergoing MRI exam for dural tears received paramagnetic contrast agents into CSF via the lumbar intrathecal space and CSF transport from subarachnoid space to cerebral PVS and into brain parenchyma was characterized over 24-48 hrs15-17. These studies reported spatial MR contrast uptake patterns from CSF into brain parenchyma similar to those observed in rodents 13,19. Other novel studies applied a range of non-invasive MR imaging techniques in combination with implementation of sleep deprivation schemes, to study CSF and water diffusivity changes during different states of arousal to explore GS function. For example, diffusion MRI imaging was used to assess changes in the apparent diffusion of water as a function of the state of sleep deprivation or arousal 28. Differences in diffusivity between awake rested, awake sleep deprived conditions and during sleep was documented that while not always consistent across studies demonstrated dynamic changes in brain parenchymal volumes that appear to be influenced by arousal 28.Acknowledgements
NIH R01AG048769, RF1 AG053991, R01AG057705 and Leducq Foundation (16/CVD/05)References
1 Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337-341, doi:10.1038/nature14432 (2015).
2 Antila, S. et al. Development and plasticity of meningeal lymphatic vessels. J Exp Med 214, 3645-3667, doi:10.1084/jem.20170391 (2017).
3 Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212, 991-999, doi:10.1084/jem.20142290 (2015).
4 Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science translational medicine 4, 147ra111, doi:10.1126/scitranslmed.3003748 (2012).
5 Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373-377, doi:10.1126/science.1241224 (2013).
6 Hablitz, L. M. et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv 5, eaav5447, doi:10.1126/sciadv.aav5447 (2019).
7 Benveniste, H. et al. Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology 127, 976-988, doi:10.1097/ALN.0000000000001888 (2017).
8 Mathiisen, T. M., Lehre, K. P., Danbolt, N. C. & Ottersen, O. P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58, 1094-1103, doi:10.1002/glia.20990 (2010).
9 Brown, R. et al. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 114, 1462-1473, doi:10.1093/cvr/cvy113 (2018).
10 Abbott, N. J., Pizzo, M. E., Preston, J. E., Janigro, D. & Thorne, R. G. The role of brain barriers in fluid movement in the CNS: is there a 'glymphatic' system? Acta Neuropathol 135, 387-407, doi:10.1007/s00401-018-1812-4 (2018).
11 Mestre, H. et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7, doi:10.7554/eLife.40070 (2018).
12 Smith, A. J., Yao, X., Dix, J. A., Jin, B. J. & Verkman, A. S. Test of the 'glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6, doi:10.7554/eLife.27679 (2017).
13 Iliff, J. J. et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123, 1299-1309, doi:10.1172/JCI67677 (2013).
14 Goulay, R. et al. Subarachnoid Hemorrhage Severely Impairs Brain Parenchymal Cerebrospinal Fluid Circulation in Nonhuman Primate. Stroke 48, 2301-2305, doi:10.1161/STROKEAHA.117.017014 (2017).
15 Eide, P. K. & Ringstad, G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open 4, 2058460115609635, doi:10.1177/2058460115609635 (2015).
16 Eide, P. K. & Ringstad, G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: A glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab, 271678X18760974, doi:10.1177/0271678X18760974 (2018).
17 Ringstad, G., Vatnehol, S. A. S. & Eide, P. K. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140, 2691-2705, doi:10.1093/brain/awx191 (2017).
18 Lee, H. et al. Quantitative Gd-DOTA uptake from cerebrospinal fluid into rat brain using 3D VFA-SPGR at 9.4T. Magn Reson Med 79, 1568-1578, doi:10.1002/mrm.26779 (2018).
19 Lee, H. et al. The Effect of Body Posture on Brain Glymphatic Transport. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 11034-11044, doi:10.1523/JNEUROSCI.1625-15.2015 (2015).
20 Benveniste, H. et al. Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology, doi:10.1097/ALN.0000000000001888 (2017).
21 Harrison, I. F. et al. Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. Elife 7, doi:10.7554/eLife.34028 (2018).
22 Jiang, Q. et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab 37, 1326-1337, doi:10.1177/0271678X16654702 (2017).
23 Ratner, V. et al. Cerebrospinal and interstitial fluid transport via the glymphatic pathway modeled by optimal mass transport. NeuroImage 152, 530-537, doi:10.1016/j.neuroimage.2017.03.021 (2017).
24 Ratner, V. et al. Optimal-mass-transfer-based estimation of glymphatic transport in living brain. Proceedings of SPIE--the International Society for Optical Engineering 9413, doi:10.1117/12.2076289 (2015).
25 Rachev, S. T. & Rüschendorf, L. Mass Transportation Problems. Vol. Volume I and II (Springer, 1998).
26 Elkin, R. et al. GlymphVIS: Visualizing glymphatic transport pathways using regularized optimal transport. Med Image Comput Comput Assist Interv 11070, 844-852, doi:doi.org/10.1007/978-3-030-00928-1_95 (2018).
27 Benamou, J. D. & Brenier, Y. A computational fluid mechanics solution to the Monge-Kantorovic mass transfer problem. Numirische Mathematik 84, 375-393 (2000).
28 Demiral, S. B. et al. Apparent diffusion coefficient changes in human brain during sleep - Does it inform on the existence of a glymphatic system? NeuroImage 185, 263-273, doi:10.1016/j.neuroimage.2018.10.043 (2019).
Figures