MR Imaging of CSF Spaces
1Neurosurgery & Hydrocephalus center, Rakuwakai Otowa Hospital, Kyoto, Japan
Synopsis
We have studied how to visualize “neurofluids” three-dimensionally by using transparency and perspective technologies with shading and texture mapping from volumetric magnetic resonance imaging. The mean volume of the intracranial cerebrospinal fluid (CSF) in healthy individuals aged ≥70 years was estimated at more than 300 mL. CSF movement with cardiac pulsation was reduced with increasing age, although the dynamics of neurofluids has not been elucidated. By developing fluid dynamics-related technology, the 3D movement of CSF can be measured quantitatively. CSF dynamic theory has been renewed; the CSF does not exhibit unidirectional bulk flow and moves in a pulsatile fashion.
Purpose
To elucidate normal aging-related changes in the volume, distribution, and movement of neurofluids, we compared healthy elderly individuals and patients with idiopathic normal pressure hydrocephalus (iNPH).Methods
The intracranial space was subdivided into the brain parenchyma and intracranial CSF spaces using a simple threshold algorithm on 3D T2-weighted sequence images on a 3D workstation. From the intracranial CSF spaces, the ventricle and three parts of the subarachnoid spaces (convexity-subarachnoid space, Sylvian fissure, and basal cistern and subarachnoid space in the posterior fossa) were manually segmented. The presence and severity of the perivascular space (PVS) in the basal ganglia (BG-PVS) and centrum semiovale (CSO-PVS) were evaluated on conventional T2-weighted or constructive interference in steady state (CISS) images after changing the window level. Using the 4D flow application of the 3D workstation, the 3D velocity (speed and direction) of the intracranial CSF was measured from the 3D phase contrast images.Results
The mean volume of the intracranial CSF in healthy individuals aged ≥60 years was more than 300 mL, whereas in patients with iNPH the volume was more than 400 mL. The volumes of the ventricles, Sylvian fissure, and basal cistern in patients with iNPH all increased, whereas that of the convexity-subarachnoid space decreased and the convex part of the brain moved upwards and was compressed (Figure 1). Intracranial CSF normally moved in a pulsatile fashion and not in a unidirectional bulk flow manner (Figure 2). The repetition rate of this movement decreased with normal aging. In patients with iNPH who had enlarged cerebral aqueduct and foramens of Luschka and Magendie, the pulsatile CSF movement in the ventricular system increased (Figure 2). BG-PVS in healthy elderly individuals was significantly associated with the presence and severity of periventricular and deep white matter hyperintensities, whereas CSO-PVS was normally visible and inversely associated with deep white matter hyperintensity (Figure 3). Conversely, CSO-PVS in patients with iNPH was sometimes invisible, especially in patients with severe periventricular white matter hyperintensity (Figure 4).Discussion
While the brain becomes smaller with normal aging, not only the CSF spaces, including the ventricle and subarachnoid space, but also the PVS enlarge. Furthermore, pulsatile CSF movement and paravascular inflow to the brain parenchyma normally reduce due to a decrease in the total volume of cerebral blood flow during aging.1 In iNPH, the CSF pathologically increases in volume due to excretory disorder of the CSF. The increasing intracranial CSF volume in iNPH opens the compensatory direct CSF pathways between the inferior horn of the lateral ventricles and basal cistern like an overflow device.2 The lateral ventricles might be more easily enlarged than the basal cistern because the subarachnoid spaces have dense partitions with trabeculae. The subarachnoid space in iNPH enlarges with a disproportionate distribution (i.e., Disproportionately Enlarged Subarachnoid-space Hydrocephalus [DESH]) which increases the volume of the basal cistern and Sylvian fissure and decreases the volume of the convexity subarachnoid space, concurrent with ventricular enlargement.3-5 Stretching of the ependymal lining by ventricular enlargement in iNPH might facilitate abnormal transependymal water transport from the lateral ventricles towards the deep white matter. According to the latest concept of the glymphatic pathway,6 CSF from the subarachnoid space is driven into the brain parenchyma through the PVS of penetrating arteries on the brain surface, and interstitial fluid in the white matter is mainly drained into the ventricles through white matter tracts or the PVS of deep cerebral veins. Based on this hypothesis, interstitial metabolic waste products would normally drain toward the ventricles rather than subarachnoid space, although the direction of flow of the interstitial fluid towards the ventricles or subarachnoid space may be complementary. Nonetheless, reduced movement and excretory disorders of the CSF might influence the convective flow of interstitial fluid. Aging-related metabolism disorder of neurofluids might be associated with a decline in cognitive function.7Conclusion
There is more than twice the volume of intracranial CSF but reciprocating motion of the CSF halves in the healthy individuals in their 70s, compared to those in their 20s. Additionally, most healthy individuals in their 70s had abundant visible CSO-PVS on conventional T2-weighted images. From now on, we must consider the dynamics and metabolism of neurofluids including not only the CSF but also the interstitial fluid.Acknowledgements
We would like to thank the staff at the department of radiology in the Rakuwakai Otowa Hospital. Additionally, we would like to thank Hiroki Itou and Jun Masumoto (FUJIFILM Corporation) and Ken Kotera (FUJIFILM Medical Corporation) for the usage of work-in-progress application in the 3D volume analyzer workstation (SYNAPSE 3D; FUJIFILM Medical Systems; Tokyo, Japan).References
1. Zarrinkoob L, Ambarki K, Wahlin A, et al. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab 2015;35:648-654
2. Yamada S, Ishikawa M, Iwamuro Y, et al. Choroidal fissure acts as an overflow device in cerebrospinal fluid drainage: morphological comparison between idiopathic and secondary normal-pressure hydrocephalus. Sci Rep 2016;6:39070
3. Yamada S, Ishikawa M, Yamamoto K. Optimal diagnostic indices for idiopathic normal pressure hydrocephalus based on the 3D quantitative volumetric analysis for the cerebral ventricle and subarachnoid space. AJNR Am J Neuroradiol 2015;36:2262-2269
4. Yamada S, Ishikawa M, Yamamoto K. Comparison of CSF distribution between idiopathic normal pressure hydrocephalus and Alzheimer disease. AJNR Am J Neuroradiol 2016;37:1249-1255
5. Yamada S, Ishikawa M, Yamamoto K. Fluid distribution pattern in adult-onset congenital, idiopathic and secondary normal-pressure hydrocephalus: implications for clinical care. Front Neurol 2017;8:583
6. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol 2018;17:1016-1024
7. Louveau A, Da Mesquita S, Kipnis J. Lymphatics in Neurological Disorders: A Neuro-Lympho-Vascular Component of Multiple Sclerosis and Alzheimer's Disease? Neuron 2016;91:957-973
Figures
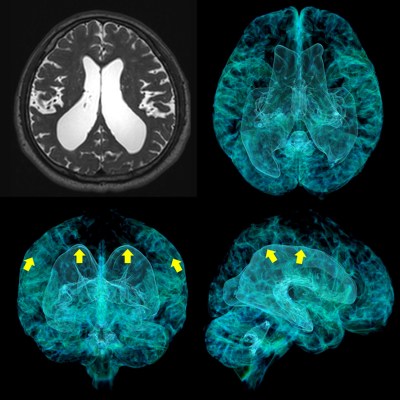
Figure 1. Disproportionately Enlarged Subarachnoid-space Hydrocephalus (DESH) in iNPH
The left upper image is a T2-weighted image. The other images are formed from the 3D T2-weighted images by using a 3D workstation. The lateral ventricles and Sylvian fissure are expanded towards the yellow arrows.
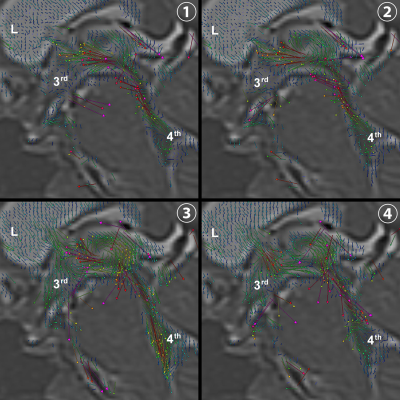
Figure 2. Three-dimensional velocity of intracranial CSF using 4D flow application
Each vector indicates the velocity of the CSF in the lateral (L), third (3rd), and fourth (4th) ventricles.
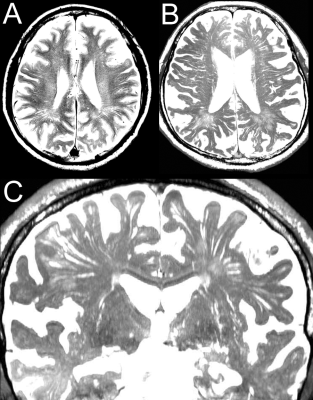
Figure 3. Perivascular space in healthy elderly individual
A - the perivascular space in the centrum semiovale (CSO-PVS) on a conventional T2-weighted image. B - the CSO-PVS on the axial view of 3D constructive interference in steady state (CISS) image. C - the CSO-PVS and perivascular space in the basal ganglia (BG-PVS) on the coronal view of the 3D CISS image.
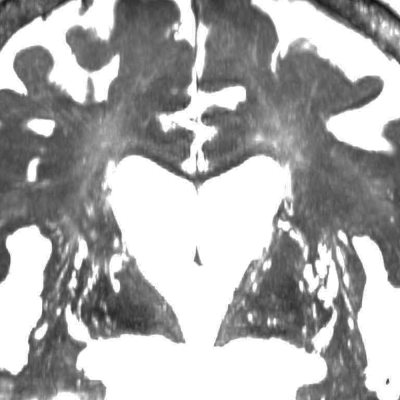
Figure 4. Perivascular space in idiopathic normal-pressure hydrocephalus (iNPH)
This figure shows the coronal view of 3D constructive interference in steady state (CISS) image for a representative case of iNPH. Many perivascular spaces in the basal ganglia (BG-PVS) were dilated but the perivascular space in the centrum semiovale (CSO-PVS) could not be seen.