Matching MR Methods With Appropriate Applications
1MRRC, Yale University, New Haven, CT, United States
Synopsis
This presentation will review the decision process to arrive at an optimal combination of MR sequence elements, such as spatial localization, water suppression and spectral editing. Examples of specific applications, metabolites and MR hardware limitations will be provided to demonstrate that an optimal choice for one condition may not necessarily be optimal for a different one. While guidelines and decision trees can help the user under most experimental conditions, an intimate knowledge of each specific MR method is required when straying off the beaten path.
Introduction
This year celebrates the 45th anniversary of the first in vivo MR spectroscopy (MRS) study performed by Hoult and coworkers (1). Since that time in vivo MRS has seen a dramatic development from one-pulse methods to extended pulse sequences employing multiple RF pulses, magnetic field gradients and delays. Today there are dozens of MR methods with colorful acronyms for spatial localization, water and/or lipid suppression and spectral editing (2). For a MR novice the range of available techniques can be overwhelming and the right choice and combination of methods may not always be obvious, especially since each technique has its own specific limitations that may or may not be important for the application at hand. Here we will discuss several decision trees that can be followed to arrive at an optimal MR pulse sequence combination for a given application, experimental setup and magnetic field strength.The challenge
Fig. 1 graphically depicts the challenge that a MR spectroscopist faces when selecting an optimal combination of MR methods for a particular application and/or metabolite. The key to successfully selecting a suitable MR method is to understand the limitations of each method together with the additional limitations posed by the available MR parameters. Fortunately, as NMR in general and in vivo MRS in particular is one of the most flexible techniques ever developed, there is typically more than one combination that can provide satisfactory results.Possible solutions
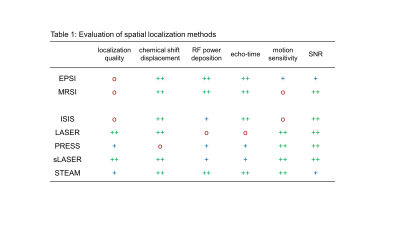
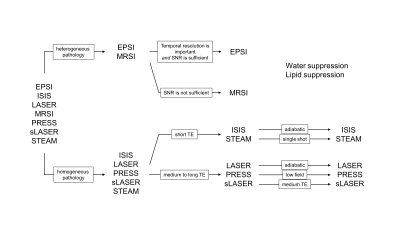
Table 1 shows a summary of several important features of available spatial localization methods. A cursory glance reveals that no single method excels at all features, so that a decision must be based on a relative balance between the various features. For example, LASER localization (3) provides excellent spatial localization, minimal chemical shift displacements and high SNR. However, LASER is rarely used in high-field human brain applications due to RF power deposition concerns and the long minimum echo-time TE. In animal studies, LASER is the preferred localization method, since short RF pulses can be employed due to the higher maximum RF amplitude, thereby giving a minimum TE of circa 10 ms. Instead of being guided purely by technical MR considerations, the decision can also be based on application-related factors (Fig. 2). When faced with a pathology that homogeneously affects specific parts of the brain (e.g. frontal cortex in many psychiatric disorders), spatial localization of a single volume may be sufficient and has the advantage of a well-defined volume definition. In the case of pathologies that affect the brain heterogeneously (e.g. cancer, multiple sclerosis) it may be better to perform MR spectroscopic imaging (MRSI) to obtain an unbiased view. Decisions further down the tree may be based on additional application-specific concerns or may be guided by MR hardware limitations. Once an optimal spatial localization method has been selected, additional decision trees can be set up for water and/or lipid suppression and selective metabolite detection through spectral editing.Conclusions
This presentation will review the decision process to arrive at an optimal combination of MR sequence elements, such as spatial localization, water suppression and spectral editing. Examples of specific applications, metabolites and MR hardware limitations will be provided to demonstrate that an optimal choice for one condition may not necessarily be optimal for a different one. While guidelines and decision trees can help the user under most experimental conditions, an intimate knowledge of each specific MR method is required when straying off the beaten path.Acknowledgements
No acknowledgement found.References
1. Hoult DI, Busby SJ, Gadian DG, Radda GK, Richards RE, Seeley PJ. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature 1974;252:285-287.
2. de Graaf RA. In Vivo NMR Spectroscopy. Principles and Techniques. Chichester: John Wiley; 2019.
3. Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson 2001;153:155-177.
Figures