Chest & Abdominal
1UW-Madison, United States
Synopsis
This syllabus and the accompanying slides serve as an introduction to the use of pulmonary MRA for the primary diagnosis of pulmonary embolism. It is our hope that you will be able to start up a program at your institution based on this information.
MRI of Venous Thromboembolic Disease
Target Audience:
This presentation is aimed at MRI trained physicians, hematologists, pulmonary medicine physicians, physicists, imaging scientists and technologists that are interested in the imaging features of acute and chronic venous thromboembolic disease.
Objectives:
Review of the history of MRI for PE (PIOPED III) and the problems with that study.
Reasons to do MRI instead of CTA or V/Q scanning.
The use of magnetic resonance angiography (MRA) for the primary diagnosis of acute and chronic pulmonary embolism.
a. The direct and indirect findings of pulmonary embolism (PE) at MRA.
b. Artifacts on MRA that may simulate PE (Gibbs’ ringing artifact).
c. The incidence of non-thrombotic findings on MRA are similar to CTA
Purpose:
To acquaint the reader/audience with the recent developments in MRI that allow for the primary diagnosis of PE and how this test can help reduce the medical radiation exposure in the population.
Background :
The PIOPED III study (1) was a prospective non randomized efficacy study of over 300 patients that compared the performance of MRA for the diagnosis of PE compared to CTA. While MRI was specific for PE it was not very sensitive (Sensitivity of 78%). The authors concluded that MRA should only be performed at centers with expertise. (1) At UW Madison we had been performing MRA for PE for over three years (starting in 2007) before these results came out. As such, our patients have been the beneficiaries of continuing to use this exam ever since. There were some important limitations to the PIOPED III study. These included a highly variable rate of good quality exams among the sites, use of only one MRA breath hold, lower resolution than is what now available and a lower relaxivity contrast agent for the first part of the study.(1-3)
The central question of the day is this one, "Why continue to beat the drum for MRA when CTA is so very good? " CTA is easy to do, readily available and easy to interpret. On the con side of this argument is the fact that MRA takes more time, is harder to schedule emergently, is more difficult to interpret, and patients have a harder time holding their breath for it. On the pro side of this argument is that there is no medical radiation, no nephrotoxicity, can be repeated using the same contrast bolus- multiple breath holds, and can be used in those patients with an iodinated contrast allergy. Building a successful pulmonary MRA program at your center is possible, but will take some education and a few clinical champions to bring it forward.(4)
Methods:
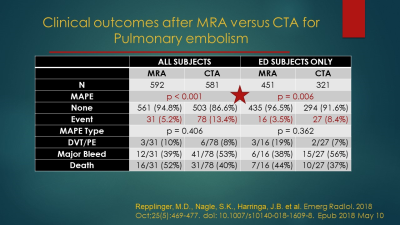
This presentation is an amalgamation of 12 years of our work using MRA for the primary diagnosis for PE in over 3000 patients. We recently published a (3) case controlled series of patients that were worked up for possible PE. We gathered over 600 cases of MRA performed for the primary diagnosis of suspected pulmonary embolism with an age and sex matched group of CTA cases. We studied patients over a ten-year period. We performed an outcomes analysis at six months for any major adverse pulmonary events (MAPE). MAPEs were defined as any deep venous thrombosis, any pulmonary embolism or death from PE within the six-month follow-up period. We also studied the origin of a low signal intensity artifact on MRA studies for PE.
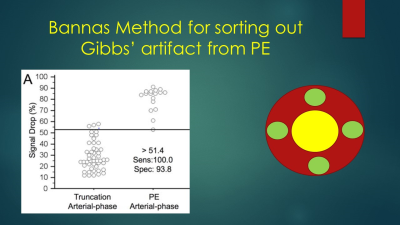
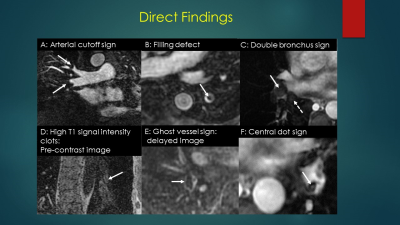
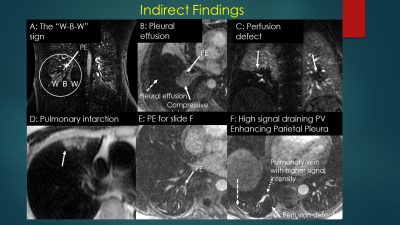
This Gibbs’ ringing artifact is a commonly found reconstruction artifact in both CTA and MRA and can often be distinguished from an embolus by using the Bannas’ method. (5) Briefly, this method uses the signal intensity value of the area of low signal intensity suspected to be an embolus and divides this value by the average of four regions of interest placed around the clot. In a review of over 1000 cases performed for the primary diagnosis of PE, we found over 50 positive cases. Two radiologists reviewed the PE positive case for the presence of direct and indirect findings of PE along with their respective inter and intra observer agreement (kappa) scores. (Benson et al, ISMRM 2019, Poster presentation) The direct findings included the following: (a) filling defect, (b) vessel cutoff, (c) high signal intensity of Methemoglobin, (d) double lumen sign, (e) ghost vessel, (f) central dot. The indirect signs we evaluated for included: (a) pleural effusion, (b) enhancing pleura, (c) pulmonary infarction, (d) white-black-white sign, (e) high intensity draining vein due to slow flow in the lung segment affected by PE.
Results: See slides
Discussion
In our twelve-year experience we have found MRA to be a reliable test for the exclusion of PE. While the resolution is not as good as CTA, this has not been measurable in terms of outcomes. We have found that a negative MRA has the same negative predictive value as a negative CTA.
Recognition of the Gibbs’ truncation artifact is critical to the accurate diagnosis of PE at MRA. Sometimes even when using the Bannas’ method it is not clear whether or not the abnormality is a PE or an artifact. In this situation, follow up with a CTA examination is necessary prior to the initiation of anticoagulation therapy.
A knowledge of the direct and indirect signs of PE at MRA is very helpful. These can be clues for the presence of smaller emboli and very large ones that are masked by underlying lung or pleural pathologies.
The appreciation of the other causes of chest pain is also crucial and can help to triage the patient for the underlying etiology of his/her complaints. Of particular interest is aortic dissection which can be easily found at MRA and can be life threatening.
Clinical reimbursement in the USA for pulmonary MRA has not been approved by the Centers for Medicare and Medicaid Services (CMS). However, if any abnormality is found on the MRI/MRA study this is often billed and collected as an MRI of the chest.
Acknowledgements
The authors wish to thank the Department of Radiology at UW-Madison and GE Healthcare for research support.References
References
1. Stein PD, Chenevert TL, Fowler SE, et al. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434-443, W142-433.
2. Schiebler ML, Nagle SK, Francois CJ, et al. Effectiveness of MR angiography for the primary diagnosis of acute pulmonary embolism: clinical outcomes at 3 months and 1 year. J Magn Reson Imaging 2013; 38:914-925.
3. Repplinger MD, Nagle SK, Harringa JB, et al. Clinical outcomes after magnetic resonance angiography (MRA) versus computed tomographic angiography (CTA) for pulmonary embolism evaluation. Emerg Radiol 2018; 25:469-477.
4. Nagle SK, Schiebler ML, Repplinger MD, et al. Contrast enhanced pulmonary magnetic resonance angiography for pulmonary embolism: Building a successful program. Eur J Radiol 2016; 85:553-563.
5. Bannas P, Schiebler ML, Motosugi U, Francois CJ, Reeder SB, Nagle SK. Pulmonary MRA: differentiation of pulmonary embolism from truncation artefact. Eur Radiol 2014; 24:1942-1949.