Abdominal Motion Detection
1Radiology, Brigham and Women's Hospital, Harvard Medical School, MA, United States
Synopsis
Sensors are everywhere, for example there are hundreds of them on a car, and they represent a natural future direction for MRI. Scanners of the future may incorporate suites of sensors gathering information about patient and hardware, providing a diverse and eclectic collection of information to be fed into convolutional neural networks for interpretation, for enhanced/improved imaging results. Work on ultrasound-based sensors will be described here in some detail; these sensors accompany patients into the MRI bore, providing streams of high-temporal resolution information about internal motion that can be utilized as part of the image reconstruction process.
Outcome/objectives:
Organ motion can be of great clinical interest (e.g., the beating motion of the heart) or merely a hindrance that limits image quality. Either way, special hardware is often needed to gather information about the motion, and special software to incorporate such information into the reconstruction process. Examples will be reviewed as part of this presentation.Purpose:
Sensors are everywhere, for example, there are hundreds of them on a car. While there are already sensors of various types on MRI scanners of course, one could imagine a future where many more would gather information about patient and hardware, and where this diverse and somewhat eclectic collection of information would be fed into convolutional neural networks or other forms of machine learning algorithms for interpretation, leading to enhanced/improved imaging results. The many sensors mentioned and described in this course do not necessarily compete with each other; in contrast, they may be destined to just take their place in future arrays of sensors that combine their signals for joint interpretation. ECG leads, respiratory bellows, pulse oximeters, RF-based sensors sensitive to the lung volume, ‘wearable’ small coils whose motion can be tracked, cameras and accelerometers all represent examples of sensors that provide information about patient and/or organ motion. In our own work we developed ultrasound-based sensors, called ‘organ configuration motion’ (OCM) sensors. These sensors, attached to the patient's skin, accompany him/her into the MRI bore and provide a stream of high-temporal resolution information about internal motion [1,2]. The MRI and OCM streams of information are combined, using machine learning, in ways that lead to enhanced imaging results. Examples where OCM sensors accompany subjects to positron emission tomography (PET) [3] or ultrasound imaging exams [4] will also be described. While other types of sensors such as cameras or accelerometers are typically sensitive to what happens at the surface of the body, OCM sensors probe its depths and as such may nicely complement other existing sensor types.Methods:
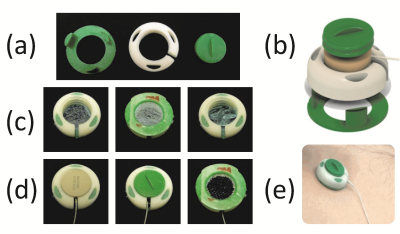
OCM sensors include a plastic capsule that was designed and 3D-printed to accommodate an MR-compatible ultrasound transducer along with ultrasound gel (Fig. 1). One side of the sensor is closed by a thin membrane essentially transparent to ultrasound energy but capable of containing the gel. The sensor is fixed to the skin, and closed by twisting a lid that pushes the transducer onto the skin to achieve proper acoustic coupling. The sensors are compact, about 3x3x1 cm in size, and a multiplexing device was developped to allow up to four sensors to be used at a time. Application to the skin simply involved peeling a protective layer and pressing the device onto the appropriate site: the abdomen for motion monitoring, or the chest for cardiac gating. Machine learning algorithms were developed to interpret and reconstruct images from the hybrid streams of OCM+MRI data that were obtained as a result [1].Results:
Up to now, hybrid OCM+MRI acquisitions have been used mostly to: help improve the temporal resolution of real-time MRI by up to two orders of magnitude and to help detect cardiac activity even at high field where magnetohydrodynamic effects might make electrocardiograms (ECGs) unreliable [1,2,5]. Other examples such as OCM+PET [3] and OCM+ultrasound imaging [4] (youtube video at https://youtu.be/TambBMGRsXM) will also be described.Conclusion:
MRI scanners of the future may include diverse suites of sensors, along with elaborate machine learning reconstruction algorithms to interpret these rich and eclectic parallel streams of data, for enhanced imaging results.Acknowledgements
Financial support from NIH grants R01CA149342, P41EB015898 and R03EB025546 is duly acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
[1] Preiswerk F, Toews M, Cheng C-C, Chiou Jr-G, Mei C-S, Schaefer LF, Hoge WS, Schwartz BM, Panych LP, Madore B. Hybrid MRI ultrasound acquisitions, and scannerless real-time imaging. Magn Reson Med 2017;78(3):897-908.
[2] Madore B, Cheng C-C, Preiswerk F. Ultrasound-based sensors for physiological motion monitoring. ISMRM 2018, Paris, France: p. 1004.
[3] Cheng C-C, Belsley G, Moore SC, Preiswerk F, Wu P-H, Foley Kijewski M, Campbell L, DiCarli MF, Madore B. Ultrasound-based sensors for motion correction of PET data. Society of Nuclear Medicine and Molecular Imaging; Philadelphia, PA, USA; 2018.
[4] Madore B, Cheng C-C, Preiswerk F. Combining MR and ultrasound imaging, through sensor-based probe tracking. ISMRM 2019, Montréal, Québec, Canada. Session: MR-Guided Intervention, Wednesday May 15th 2019, Room 513D-F.
[5] Preiswerk F, Cheng C-C, Wu P-H, Panych LP, Madore B. Ultrasound-based cardiac gating for MRI. ISMRM 2017, Honolulu, USA: p. 4443.
Figures