Physics & Chemistry of d-DNP for Biomedical Imaging
1A.I.Virtanen Institute for Molecular Sciences, University of Eastern Finland, Kuopio, Finland
Synopsis
Dissolution dynamic nuclear polarisation (d-DNP) method allows hyperpolarisation of a broad range of molecules, yielding >10,000-fold increase in 13C signal-to-noise ratio. Hyperpolarisation is achieved through microwave-induced polarisation transfer from electrons to nuclei at solid-state followed by rapid dissolution. d-DNP places some limitations to suitable marker molecules, however. In this talk, the basics of d-DNP and chemistry involved will be discussed.
OUTCOME/OBJECTIVES
Low sensitivity is an inherent feature of MR, for example at 7T and room temperature (300K) proton and carbon polarisations are only 24 ppm and 6 ppm (estimated using Boltzmann equation), respectively. Dissolution dynamic nuclear polarisation (d-DNP) is currently the most versatile tool to achieve hyperpolarised molecules, for example yielding >10,000-fold increase in 13C signal-to-noise ratio (1, 2). In this talk, the basics of the technique are described.METHODS
Theoretical and experimental basis of dynamic nuclear polarisation was developed already more than 50 years ago (see for example (3) for a more detailed explanation of theoretical basis of DNP). For DNP technique, an external hyperpolariser magnet, operating at high magnetic field (>3T) and equipped with variable temperature insert operating at <1.5K, is currently needed. When a sample containing a marker molecule (e.g. 13C-labelled metabolite although the method is suitable also for other nuclei) and a radical with unpaired electrons is placed inside hyperpolariser, the 13C polarisation increases to 1285 ppm at 7T (Figure 1). More importantly, in these conditions electrons are nearly 100% polarised. Microwave-irradiation at close to electron Larmor frequency can be used to induce polarisation transfer between electrons and nearby nuclei in the sample. In solid-state, spin-diffusion will disperse the gained polarisation throughout the sample and long nuclear T1 relaxation times lead to amplification of signal over time, typically from 0.5-2h, until a new hyperpolarised steady-state (even up to 100%) is obtained. In 2003, Ardenkjaer-Larsen et al. demonstrated that a rapid dissolution of the solid sample allows retention of this hyperpolarisation (1). This opened up a way for removing the sample as a liquid that could be subsequently used in a normal imaging magnet within the lifetime of hyperpolarised signal (~five times the liquid-state T1 relaxation time. It is also important to remember that every RF excitation irreversibly uses some of the hyperpolarised signal). Importantly, the field strength of the imaging magnet is not so critical because the sample has been prepolarised. The d-DNP technique typically yields 10-70% (100,000-700,000 ppm) 13C polarisation levels at room temperature after dissolution. For comparison, it would take a magnet operating at >100,000 T to achieve the same without d-DNP.
Due to its relatively simple basic principle, d-DNP technique is very flexible and allows hyperpolarisation of a wide range molecules with small molecular weight. However, a molecule needs to fulfill some criteria to be a suitable candidate for d-DNP and in vivo use.
1) A long liquid-state T1 allow the retention of hyperpolarisation for as long as possible. In case of 13C, this means that carboxyl groups with no directly-bonded protons are most commonly used because they have T1 times of >10s (e.g. in pyruvate 60-70s) (4). Contrast this to methyl groups, typically used for non-DNP 13C spectroscopy, with T1 of 1-2s although deuteration can extend this in some cases. From a biological point of view, fast, mainly catabolic, enzymatic reactions are therefore most accessible to d-DNP techniques.
2) Dissolution step usually leads to a significant sample dilution because dissolution needs to occur rapidly to retain hyperpolarisation. Therefore the molecule needs to be readily soluble (>1M) in the original sample solution. For example, the most commonly used d-DNP molecule, pyruvic acid, is 14M before dissolution and 80-250mM after dissolution. Efficient polarisation transfer requires that radical are dispersed equally inside an amorphous structure, therefore aqueous samples often need an added glassing agent (e.g. glycerol). The solubility of marker molecule needs to be retained also in these conditions.
3) The most interesting aspect of d-DNP studies is the possibility to follow enzymatic or chemical reactions in real-time. To achieve this, the labelled nuclei must have sufficient chemical shift difference between the precursor molecule and its products.
4) Despite large gains in sensitivity, injected concentrations are still high (e.g. 80 mM and 5-10ml/kg for preclinical use) and often supraphysiological. The toxicity of the molecule needs to therefore be taken in to account.
5) For most biological uses, a molecule with limited metabolism in blood is usually preferred so that the majority of the metabolism will happen only in the target tissue.
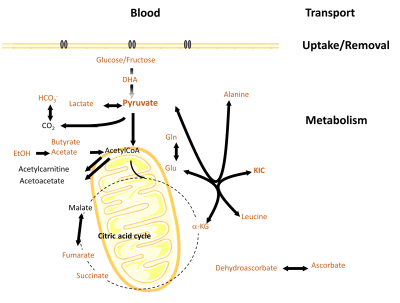
A large number of biologically relevant metabolites fulfil the above criteria and have been demonstrated suitable for d-DNP use both in vitro and in vivo (Figure 2).
CONCLUSION
The advances in d-DNP have paved way for MR-based real-time metabolic imaging. A number of important applications have been already identified in the fields of oncology and cardiac research, and the method is currently undergoing clinical translation (5, 6). A better understanding of the underlying physics and chemistry will allow for further increases in polarisation levels and faster polarisation times.Acknowledgements
The d-DNP research in Kuopio has been funded by the Academy of Finland and European Regional Development Fund (ERDF).References
1. Ardenkjaer-Larsen, et al. (2003) Proc Natl Acad Sci U S A, 100(18): 10158-63.
2. Ardenkjaer-Larsen (2018) eMagRes, doi: 10.1002/9780470034590.emrstm1549
3. Wenckebach (2016) Essentials of Dynamic Nuclear Polarization, ISBN, 907554118X, 9789075541182.
4. Keshari and Wilson (2014) Chem Soc Rev, 43(5):1627-1659.
5. Kurhanewicz et al. (2019) Neoplasia, 21(1):1-16.
6. Wang, et al. (2019) Radiology, doi: 10.1148/radiol.2019182391.
Figures