Diffusion & Perfusion Weighted Imaging
1Dept. of Biomedical Engineering, University of Basel, Allschwil, Switzerland, 2Dept. of Radiology, Radiological Physics, University Hospital Basel, Basel, Switzerland
Synopsis
This lecture will explain the two important and popular imaging concepts of “diffusion weighted imaging (DWI)” and “perfusion weighted imaging (PWI)”. The underlying physics and fundamental properties will be explained in a pictorial way (with only a few easy mathematical equations that may be important to recognize or use). The clinical significance and potentials of the two methods are also discussed. At last, DWI and PWI are combined to establish the so-called “diffusion-perfusion-mismatch-concept” in (acute) ischemic stroke.
Introduction
This lecture will shed some first light on the two imaging concepts of diffusion weighted imaging (DWI) and perfusion weighted imaging (PWI). On the one side, one may criticize that for the lecture time given, such a presentation will definitely be an incredible or even impossible task: To give sufficient credit to both of these important imaging concepts. On the other side, one could say: "Come on, let's talk about the basic ideas of the two and why they often appear to be like siblings -- although they are two very different effects." Well, this syllabus follows the latter argument :-).
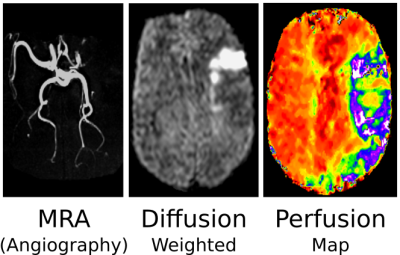
Until a few years ago, the ISMRM society had a single study group called the diffusion and perfusion study group. The main reason is/was that "diffusion" as an imaging method got its first clinical significance in combination with perfusion imaging for the so-called diffusion-perfusion-mismatch-concept in (acute) ischemic stroke (1-4): The pivotal question after an acute ischemic stroke is whether there exists so called "tissue at risk" -- tissue, which is impaired by a lack of blood supply but not irreversibly damaged, yet. Such tissue will most probably benefit from a fibrinolysis in order to achieve a reperfusion of the affected vessels. A short example is presented as a teaser in Fig.1. Here, diffusion imaging represents an early marker for the viability of tissue cells, perfusion imaging represents a means to quantify tissue blood supply. The resulting area mismatch suggests "tissue at risk". The concept will be explained in further detail in the lecture based on the fundamental properties of diffusion and perfusion. These fundamental properties also represent the topic of the following pages of this syllabus.
Diffusion
The Basic Physical Effect ...
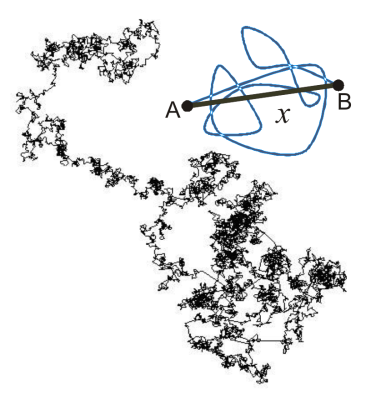
Actually, "diffusion" is a physical effect that is almost pervasive -- yet, we just do not realize it, do not think about it or we simply do not know it. For instance, anyone who ever made tea in his or her life already encountered diffusion. After brewing up, "tea" comes into existence round the leaves. Then these cords of tea turn up in the surrounding pure water and after a while of steeping there is "just tea". Observing this fascinating effect a bit more from a physical point of view, we recognize that with the steeping of tea leaves two phases become present, pure water and tea, which mix themselves "automatically" within the time. The cause or reason for this is diffusion -- also known as Brownian motion of (water) molecules. What we, i.e. human beings, and other creatures perceive as temperature is in the factual world of physics nothing else but motion, rotation and vibration of atoms and molecules, observed on a microscopic scale. The higher the temperature is the faster the atoms and molecules move, rotate and vibrate in gases, liquids and even solids. But, let's keep the gases and solids aside here and stick to water and watery human tissue.In our previous tea example -- and this tea is really hot -- the water molecules are in very fast motion (besides rotation and vibration). As a consequence, the two phases (pure water and newly existing tea) interfuse more and more until there is a uniform distribution of "water-tea", which is the final tea we want.But why does it take a while? Admittedly, the molecules are quite small, but they are also very fast ... and close together. Thus, they constantly bump into each other, about 10^21 times a second, and so they constantly change their direction and speed after these collisions. A closer look at a single molecule reveals that it performs a random walk of independent random steps over time, e.g. as shown in Fig.2. Such trajectories are not deterministic due to the stochastic nature of the diffusion process. The mean displacement, however, can be calculated, which is the average net distance of the particles from their origin. Water can diffuse freely and isotropically in big pools, and approximately in a cup of tea (free isotropic diffusion). In the case of this free isotropic diffusion the Einstein-Smoluchowski relation quantifies the average net displacement <x> after the expired time Δ (Fig.2):
[1] <x> = SQRT(2*n*D*Δ) ,
with n=1,2,3 being the number of spatial dimensions. Δ is usually called the diffusion time. With increasing diffusion time Δ the mean displacement <x> increases.
The proportionality factor D is the diffusion coefficient. It is a measure for the mobility of the diffusing molecules in the medium and it significantly depends on the temperature. In MRI, its unit is usually mm^2/ms. Representative values for 'normal' water (H2O) are DH2O (20°C) = 0.002 mm^2/ms and DH2O (37°C) = 0.003 mm^2/ms.
In tissue, water molecules demonstrate the same fundamental behavior; they are in permanent diffusional motion due to their thermal energy. But is it free diffusion? No! The existence of compartments such as extracellular spaces, intracellular spaces, intravascular spaces and cell membranes restrict diffusion (restricted diffusion). Thus, the characteristics of diffusional motion in tissue may considerably differ from the characteristics of diffusional motion in pure liquid. As a first consequence, the observed diffusion coefficients in soft tissue are lower than for free water diffusion. For example, it is DGM (37°C) = 0.0008 mm^2/ms for cerebral gray matter (GM).
For very short diffusion times tissue diffusion may still be regarded as quasi free, however, for longer diffusion times more and more molecules will collide with barriers: Then diffusion is not free, Eq.[1] is not valid anymore.Diffusion in biological tissue is not only restricted but also often anisotropic, i.e. its magnitude depends on the direction. Typical examples are orientated micro structures like fiber bundles in cerebral white matter (WM). Diffusion parallel to such a fiber bundle is almost free, whereas diffusion perpendicular to the fiber bundle is considerably restricted due to the confinement of the fibers' membranes: D|| WM (37°C) = 0.002 mm^2/ms, D⊥ WM (37°C) = 0.0002 mm^2/ms. The most common mathematical representation in MRI to account for this effect is the diffusion tensor.
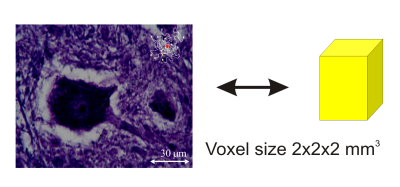
As we have seen, diffusion depends on restrictions, anisotropy, diffusion times, and a multitude of other factors that have not been mentioned, yet. Thus, any experiment will measure an average diffusion effect, since diffusion takes place on a microscopic scale but the voxel size of MRI diffusion measurements is approximately between 1mm and 3mm. Hence, any measured diffusion coefficient is usually called an apparent diffusion coefficient ADC, implying that a multitude of factors influences the measured parameter in a voxel, Fig.3. This challenge is at the same time also a major reason for the fascination that users perceive in diffusion imaging methods: Diffusion is a very sensitive marker for morphological and functional (metabolic) changes on the cellular level. It gives a deep insight into tissue micro structure.
How to Image it ...
Clinical MRI images the presence of hydrogen nuclei (protons) in the tissue. Thus, MR imaging methods based on diffusion weighting will be particularly sensitive to the diffusion of water molecules in the biological tissue. -- How does it work?
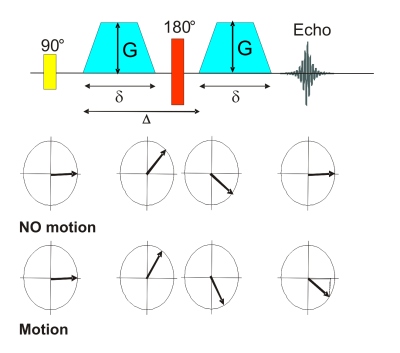
So called diffusion imaging pulse sequences use motion sensitizing gradients to produce signal attenuation which is directly related to the present diffusion coefficient in the tissue. The most popular diffusion preparation experiment is the pulsed gradient spin echo (PGSE) or so called Stejskal-Tanner sequence, Fig.4. The original PGSE sequence consists of a conventional spin echo with two symmetric, monopolar gradients added. Without motion, the phase shift introduced by each gradient is the same, but with opposite sign. Thus, they compensate each other. With motion present, a net phase shift results that is proportional to the speed of the spin. Actually, this effect is also exploited in phase contrast based flow measurement techniques. Whereas there a coherent macroscopic flow is imaged, which results in a net phase shift of the total magnetization vector, the stochastic nature of diffusion causes different phase shifts for each spin. A diffusion correlated dephasing of the magnetization results, i.e. signal attenuation. The signal attenuation is therefore directly diffusion dependent. The dephasing caused by the diffusion is irreversible.
The diffusional signal attenuation is usually described by a mono-exponential decay in the simplest case:
[2] SI(b) / SI0 = exp(-b*D) ,
where D signifies the diffusion coefficient, S(b) the signal measured with diffusion weighting gradients turned on and S0 the signal without any diffusion weighting gradients.
The new parameter b, called the b-factor, depicts the quantitative diffusion sensitivity of a DWI sequence in MRI. Its units are the inverse of D, thus ms/mm^2.
For a PGSE sequence with the common simplification of ideal, rectangular diffusion gradient pulses of constant amplitude g and duration δ and with the temporal separation of Δ the resulting b-factor is:
[3] b = γ2 g2 δ2 (Δ - δ/3) ,
where γ is the proton gyromagnetic ratio.
Any gradient in MRI, and therefore also any diffusion weighting gradient, posesses a defined direction in the coordinate system. As a result, diffusion weighting only takes place parallel to that direction. For an isotropically weighted diffusion image, hence, three images with perpendicular diffusion weighting have to be acquired and then combined.
For the read-out of the diffusion weighted echoes usually single-shot echo planar imaging (EPI) sequences are employed in order to produce diffusion weighted images.
Diffusion Applications
Today, common applications for DWI include:
- Diffusion-perfusion-mismatch for stroke imaging.
- Early detection / screening: Changed ADC due to cellularity or metabolism changes in metastases and micro fractures, for instance.
- Differential diagnosis: Differentiation of various pathologies by means of DWI and ADC, e.g. differentiation of benign and malign tumors.
- Monitoring of therapies: Nekrotic vs. vital tissue etc.- Clinical basic research: Metabolism, structure, aging of cells etc.
Perfusion
What is it?
Blood flow through the vasculature supplies the tissue with nutrients and oxygen in the human body. Thus,blood supply itself is an important measure for the vitality of tissue and it represents a key parameter. The knowledge of the dynamic properties of blood supply are of great diagnostic value.
One important parameter is the regional blood volume (RBV), which describes the subvolume occupied by blood within a volume of interest or in 100g of tissue. This, basically, is equivalent to defining it as the percental volume fraction of blood in the tissue:
[4] RBV = Vblood / Vvoxel .
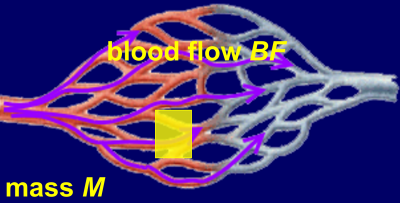
In the brain, the RBV is usually denoted as the cerebral blood volume (CBV). Unfortunately, the arbitrary units of the MRI signal do not allow a direct measurement of the RBV or CBV. Blood supply of tissue is quantified via the parameters blood flow rate BF and perfusion P. In literature, these terms are often loosely defined as 'blood flow' or equivalent. It is (c.f. Fig.5) :
[5] P = BF / M .
M is the tissue mass in 100g. Thus, compared to the blood flow rate BF the perfusion P is a mass-specific parameter. The latter makes sense, because absolute blood flow rates have a quite limited value without knowing the mass of reference. For example, organs have quite different sizes. BF is measured in milliliters of blood per minute (mL/min), therefore, P has the nominal unit milliliters of blood per minute per 100g (mL/min/100g).
To have some values for the human brain at hand: In healthy tissue the average brain perfusion equals to (50-60) mL/min/100g. However, it has to be considered that the brain perfusion is quite different from region to region. For example, the mean perfusion in gray matter is approximately (80-130) mL/min/100g, and 20 mL/min/100g in white matter.
Another important parameter that depicts the dynamic properties of tissue blood supply is the mean transit time (MTT). MTT is the average time required for a given tracer to pass through the tissue. As a rule of thumb, for agents that remain in the blood the MTT is typically a few seconds.
There is a direct relation between the parameters RBV, BF, and MTT for perfusion measurements using a contrast agent in MRI:
[6] MTT = RBV / BF .
The MTT parameter is important since it demonstrates a very sensitive contrast between ischemic and healthy tissue, for instance.
How to Image it ...
In order to probe the properties of the blood circuit, tracers are injected into the blood and their passage is observed at a point or region of interest. For MRI, contrast agents (CA) that induce a strong (local) T1 and T2 relaxation time change are employed. Contrast agents also build up significant susceptibility effects.
There are two basic CA based measurement methods in the clinics:
- Dynamic contrast enhanced (DCE) perfusion MRI utilizes the strong T1 shortening of contrast agents. The local presence of a CA leads to a strong signal enhancement in fast RF spoiled gradient echo (FLASH) sequences.
- Dynamic susceptibility contrast (DSC) perfusion MRI utilizes the considerable T2 shortening of contrast agents and their strengthening of susceptibility effects around vessels. Thus, after CA administration, a significant drop in the T2* weighted signal of a fast GE-EPI sequence is observed in the signal-time-course (bolus measurement).
Acknowledgements
No acknowledgement found.References
1. Schlaug G, Benfield A, Baird AE, Siewert B, Lövblad KO, Parker RA, Edelman RR, Warach S. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology 1999;53:1528–1537.
2. Petrella JR, Provenzale JM. MR Perfusion Imaging of the Brain. American Journal of Roentgenology 2000;175:207–219. doi: 10.2214/ajr.175.1.1750207.
3. Kane I, Carpenter T, Chappell F, Rivers C, Armitage P, Sandercock P, Wardlaw J. Comparison of 10 Different Magnetic Resonance Perfusion Imaging Processing Methods in Acute Ischemic Stroke Effect on Lesion Size, Proportion of Patients With Diffusion/Perfusion Mismatch, Clinical Scores, and Radiologic Outcomes. Stroke 2007;38:3158–3164. doi: 10.1161/STROKEAHA.107.483842.
4. Baron J-C. Perfusion Thresholds in Human Cerebral Ischemia: Historical Perspective and Therapeutic Implications. Cerebrovasc Dis 2001;11:2–8. doi: 10.1159/000049119.
Figures