High Field Imaging
1High-Field and Hybrid MR Imaging, Erwin L. Hahn Institute for MRI, University Essen-Duisburg, Essen, Germany
Synopsis
With more than 80 installed MRI systems worldwide operating at a magnetic field strength of 7 Tesla or higher, ultra-high field (UHF) MRI has been established as a platform for clinically oriented research in recent years. Profound technical and methodological developments have helped to overcome the inherent physical challenges of UHF radiofrequency (RF) signal homogenization in the human body. The ongoing development of dedicated transmit/receive RF coil arrays was pivotal in realizing UHF body MRI, beyond mere brain imaging applications. Against this backdrop, UHF MRI recently has demonstrated capabilities and potentials for clinical diagnostics in a variety of studies.
Target Audience
Clinicians and physicists interested in ultra-high field (UHF) MRI.
Objectives
To understand the motivation, physical challenges and basic technical solutions to perform UHF MRI. To review recent clinical applications of UHF MRI in neurovascular, abdominal and pelvic MR imaging.
High Field Imaging
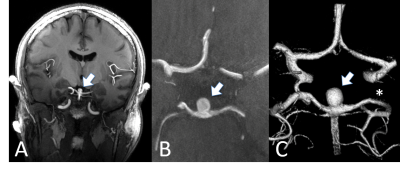
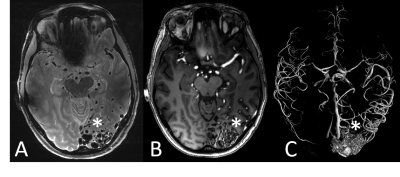
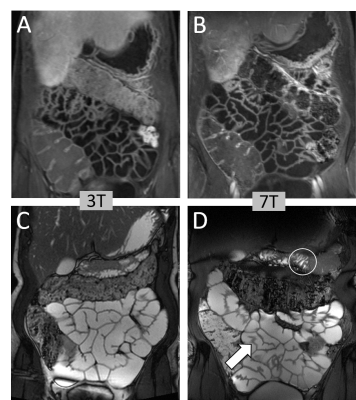
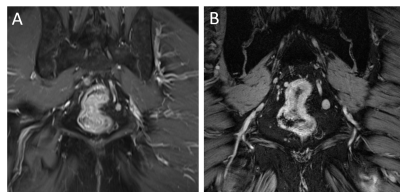
With more than 80 installed magnetic resonance imaging (MRI) systems worldwide operating at a magnetic field strength of 7 Tesla (7 T) or higher, ultra-high field (UHF) MRI has been established as a platform for clinically oriented research in recent years [1]. The driving force behind all hardware and methods developments for UHF MRI is the exploitation of the inherently higher signal-to-noise ratio (SNR), the pivotal currency in MR. In a recent study on SNR and MR tissue parameters in human brain imaging at 3, 7, and 9.4 T experimental evidence of a supralinear increase of SNR with field strength (SNR ∼ B01.65) was shown [2]. Profound technical and methodological developments have helped to overcome the inherent physical challenges of UHF radiofrequency (RF) signal homogenization in the human body. The general advantages and challenges for imaging at ultra-high field strength have been expertly described in many previous reviews [1, 3-9]. The ongoing development of dedicated transmit/receive RF coil arrays was pivotal in realizing UHF body MRI, beyond mere brain imaging applications [10]. Against this backdrop, UHF MRI recently has demonstrated capabilities and potentials for clinical diagnostics in a variety of studies. Owing to numerous methodological and hardware developments, the UHF MRI community has witnessed the expansion of the application range from brain [11] and musculoskeletal imaging [12] to first body MR imaging applications [13]. For neuro applications, the increase in SNR and the enhanced sensitivity to magnetic susceptibility have allowed a more detailed depiction of microvascularity, and high-resolution imaging of cerebral vessel pathologies, which offer potential benefits for patient treatment in terms of diagnostics, surgical planning, and therapy monitoring (Fig. 1, 2) [14, 15]. For UHF body MRI applications, recent methodological developments regarding multi-channel body RF coils, RF shimming and parallel transmit strategies, and SAR supervision are just now enabling the exploitation of the potential of UHF MR imaging. Despite these significant physical and methodological challenges, impressive body UHF MRI applications have been demonstrated for abdominal and pelvic imaging (Fig. 3, 4) [13, 16, 17, 18]. High-resolution, high contrast cancer imaging of the breast, the prostate, the cervix and rectum at 7 T magnetic field strength may provide a diagnostic advantage in oncologic imaging over lower field strength MRI [18].
Acknowledgements
No acknowledgement found.References
1. Kraff O, Quick HH. 7T: Physics, safety, and potential clinical applications. J Magn Reson Imaging. 2017 Dec;46(6):1573-1589.
2. Pohmann R, Speck O, Scheffler K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 Tesla using current receive coil arrays. Magn Reson Med 2016;75:801–809.
3. Kraff O, Fischer A, Nagel AM, Monninghoff C, Ladd ME. MRI at 7 Tesla and above: demonstrated and potential capabilities. J Magn Reson Imaging 2015;41:13–33.
4. de Boer A, Hoogduin JM, Blankestijn PJ, et al. 7 T renal MRI: challenges and promises. Magn Reson Mater Phys 2016;29:417–433.
5. Balchandani P, Naidich TP. Ultra-high-field MR neuroimaging. Am J Neuroradiol 2015;36:1204–1215.
6. Trattnig S, Bogner W, Gruber S, et al. Clinical applications at ultrahigh field (7 T). Where does it make the difference? NMR Biomed 2016;29:1316–1334.
7. Moser E, Stahlberg F, Ladd ME, Trattnig S. 7-T MR—from research to clinical applications? NMR Biomed 2012;25:695–716.
8. Webb AG, Van de Moortele PF. The technological future of 7 T MRI hardware. NMR Biomed 2016;29:1305–1315.
9. Ladd ME, Bachert P, Meyerspeer M, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018 Dec;109:1-50.
10. Rietsch SHG, Orzada S, Maderwald S, Brunheim S, Philips BWJ, Scheenen TWJ, Ladd ME, Quick HH. 7T ultra-high field body MR imaging with an 8-channel transmit/32-channel receive radiofrequency coil array. Med Phys. 2018 Jul;45(7):2978-2990.
11. Gizewski ER, Monninghoff C, Forsting M. Perspectives of ultra-high-field MRI in neuroradiology. Clin Neuroradiol 2015;25(Suppl 2):267–273.
12. Trattnig S, Zbyn S, Schmitt B, et al. Advanced MR methods at ultrahigh field (7 Tesla) for clinical musculoskeletal applications. Eur Radiol 2012;22:2338–2346.
13. Wrede KH, Matsushige T, Goericke SL, et al. Non-enhanced magnetic resonance imaging of unruptured intracranial aneurysms at 7 Tesla: Comparison with digital subtraction angiography. Eur Radiol 2017;27: 354–364.
14. Wrede KH, Dammann P, Johst S, et al. Non-enhanced MR imaging of cerebral arteriovenous malformations at 7 Tesla. Eur Radiol 2016;26: 829–839.
15. Hahnemann ML, Kraff O, Orzada S, et al. T1-weighted contrast enhanced magnetic resonance imaging of the small bowel: comparison between 1.5 and 7 T. Invest Radiol 2015;50:539–547.
16. Hahnemann ML, Kraff O, Maderwald S, et al. Non-enhanced magnetic resonance imaging of the small bowel at 7 Tesla in comparison to 1.5 Tesla: First steps towards clinical application. Magn Reson Imaging 2016;34:668–673.
17. Laader A, Beiderwellen K, Kraff O, et al. 1.5 versus 3 versus 7 Tesla in abdominal MRI: A comparative study. PLoS One. 2017 Nov 10;12(11):e0187528.
18. Philips BWJ, Fortuin AS, Orzada S, Scheenen TWJ, Maas MC. High resolution MR imaging of pelvic lymph nodes at 7 Tesla. Magn Reson Med. 2017 Sep;78(3):1020-1028.
Figures