Bringing It All Together: Relaxation, T1rho, T2rho & CEST
Synopsis
Spin-lock experiments employ RF to probe dynamic processes in molecules. CEST also employ RF to create contrast and explore spin dynamics. In all these cases, in the presence of RF, an effective field is formed and the relaxation processes are governed by the constants parallel and perpendicular to the effective field – T1rho and T2rho. We will dwell in more details on the basic principles, equations and features governing relaxation and dynamics processes in these methods.
Target Audience
Researchers interested in more in-depth understanding of relaxation and dynamics in the presence of RF. The discussion will focus on the principles behind T1rho (spin-lock), T2rho and CEST experiments.Objectives
The presentation will discuss the following concepts and connections between them:
- Relaxation
- RF influence and rotating frame relaxation
- T1rho and T2rho
- Relaxation and dynamics in CEST
- CEST and T1rho
Introduction
Relaxation is one of the most basic concepts in Magnetic Resonance, yet one of the most complicated. Under influence of RF, relaxation constants, T1rho and T2rho, are derived. Spin-lock experiments employ RF to probe dynamic processes in molecules. CEST (and MT) also employ RF to create contrast and explore spin dynamics. However, the similarity between CEST and T1rho imaging experiments is often overlooked. In this educational, we will dwell in more details on the basic principles, equations and features governing relaxation and dynamics in these methods.Theory
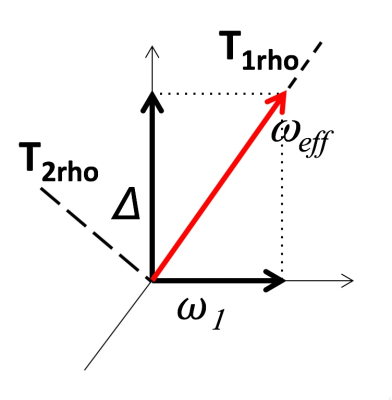
In Magnetic Resonance experiments two relaxation constants are defined [1]: longitudinal (T1) and transverse (T2). In the presence of RF, an effective field is formed, as depicted in Figure 1 [2, 3]. Thus, the relaxation processes are governed by the constants parallel and perpendicular to the effective field – T1rho and T2rho [4]. Note, that in the off-resonance T1rho experiments the effective field is a combination of off-resonance (Δ) and RF intensity (ω1), as depicted in Figure 1: ωeff=(ω21+Δ2)1/2. For dynamic processes with rates close to ωeff the measurements are influenced by the dynamics of the spin-system. Thus, experiments measuring and analyzing T1rho (or spin-lock experiments) had been employed to study molecular dynamics [1, 3, 5] and were translated into imaging to study dynamics processes in-vivo [6].
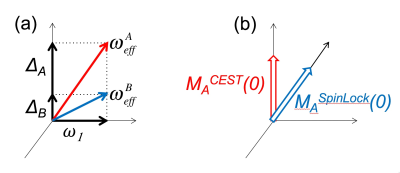
When two (or more pools) are involved, with magnetization exchange between them (mediated via chemical or dipolar interaction), the precise depiction of dynamics is more complicated. The schematic of the effective fields for two pools (A and B) is shown in Figure 2a, for the case when RF intensity is lower than the chemical shift difference (ΔΑΒ>ω1). CEST and off-resonance spin-lock are very similar experiments [7], with the main difference in the initial state of the magnetization. The initial magnetization is aligned with the effective field in the case of spin-lock experiments, or Z axis in the case of CEST (Figure 2b). The on-resonance T1rho experiment might be more sensitive to the fast-to-intermediate exchange regime, while CEST and off-resonance T1rho are more applicable in the slow exchange regime [7].
Effective fields and corresponding relaxation rates can be derived for more complex RF scenarios, such as adiabatic pulses[8] or periodically applied pulses[9, 10].
Conclusion
Employing RF to study molecular dynamics is versatile methodology. Exploring basic principles, similarities and differences between various techniques helps optimizing the approaches and may lead to development of new contrast mechanisms.Acknowledgements
No acknowledgement found.References
1. Ernst, R.R., G. Bodenhausen, and A. Wokaun, Principles of Nuclear Magnetic Resonance in One and Two Dimensions, 1994, Clarendon Press: Oxford.
2. Boulat, B. and G. Bodenhausen, Cross Relaxation in Magnetic Resonance: An Extension of the Solomon Equations for a Consistent Description of Saturation.Journal of Chemical Physics, 1992. 97: p. 6040-6043.
3. Palmer, A.G., Chemical exchange in biomacromolecules: Past, present, and future.Journal of Magnetic Resonance, 2014. 241: p. 3-17.
4. Hartmann, S.R. and E.L. Hahn, Nuclear double resonance in the rotating frame.Physical Review, 1962. 128: p. 2042.
5. Cavanagh, J., et al., Protein NMR Spectroscopy. 2007: Elsevier Academic Press.
6. Santyr, G.E., R.M. Henkelman, and M.J. Bronskill, Spin locking for magnetic resonance imaging with application to human breast.Magnetic Resonance in Medicine, 1989. 12(1): p. 25-37.
7. Jin, T., et al., Spin-locking versus chemical exchange saturation transfer MRI for investigating chemical exchange process between water and labile metabolite protons.Magnetic resonance in medicine, 2011. 65(5): p. 1448-1460.
8. Michaeli, S., et al., T1 rho MRI contrast in the human brain: Modulation of the longitudinal rotating frame relaxation shutter-speed during an adiabatic RF pulse.Journal of Magnetic Resonance, 2006. 181(1): p. 135-147.
9. Rhim, W.-K., D.P. Burum, and D.D. Elleman, Multiple-Pulse Spin Locking in Dipolar Solids.Physical Review Letters, 1976. 37(26): p. 1764-1766.
10. Zhang, S., et al., Balanced Steady-State Free Precession (bSSFP) from an effective field perspective: Application to the detection of chemical exchange (bSSFPX).Journal of Magenetic Resonance, 2017. 275: p. 55-67.
Figures