4783
A Deep Learning Algorithm for Non-Cartesian Coil Sensitivity Map Estimation1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Engineering Physics, Tsinghua University, Beijing, China, 3Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
The use of parallel imaging (PI) to exploit the encoding power of multiple coil sensitivity patterns is essential for any modern method for accelerating MRI. In practice, the need to estimate sensitivity maps when using an image-space PI formulation delays the image reconstruction process, particularly for non-Cartesian acquisitions. This paper presents a deep learning method to estimate sensitivity maps from non-Cartesian dynamic imaging data. Results show that this algorithm provide a significant reduction in the time (from 42s to 2.5s for 12 coils) for generating high-quality coil sensitivity maps from non-Cartesian MR data compared to the conventional algorithms.
Introduction
The use of parallel imaging (PI) to exploit the encoding power of multiple coil sensitivity patterns1-3 is a standard component of any modern method for accelerating MRI. In practice, the need to estimate sensitivity maps when using an image-space (or “SENSE-like”2) PI formulation delays the image reconstruction process, particularly for non-Cartesian acquisitions. Deep learning has recently been shown to be useful for fast estimation of sensitivity maps for Cartesian brain imaging4. Here we propose and demonstrate a deep learning method to estimate sensitivity maps from non-Cartesian dynamic cardiac imaging data, evaluating the impact of the estimated sensitivity maps on MR multitasking5 image reconstruction.Method
The proposed deep learning network generates high-quality coil sensitivity maps Sout from low-quality initial sensitivity map estimates Sin. The Sin were chosen as the regridded coil images divided by their sum-of-squares combination (“regridded SOS”), which can be quickly calculated but which contain artifacts and spatially varying noise levels. The real and imaginary parts of each map were separated and fed through a modified U-net6 which incorporated a residual network (ResNet7) module applied to every convolutional layer block to improve training speed. The network structure is illustrated in Figure 1.
The network was trained using a three-part loss function: mean squared error (MSE) loss, regularization loss, and smoothing loss. The MSE loss is directly calculated from the mean squared error between the output sensitivity maps and the labeled sensitivity maps generated using conventional methods8,9. The regularization loss calculates the L2-norm of all parameters in the network to reduce overfitting. The smoothing loss takes the smoothness of sensitivity maps into consideration by calculating the spatial gradients of the output sensitivity maps in each direction then calculating the resulting L2-norm (Figure 2). The smoothing loss works as a constraint on coil sensitivity physics and is key for generating high quality sensitivity maps. The neural network was implemented in TensorFlow 1.10 and trained with Adam Optimizer. Data augmentation such as random flip and random crop were implemented to provide more data for training.
All data were acquired on 3T Siemens Verio and Biograph mMR systems using the modified golden-angle radial sampling pattern designed for MR multitasking5; each dataset contained data from twelve to eighteen body coils. 2000 datasets were used for training and 1000 datasets were used for testing.
Results
Figure 3 shows the magnitude and phase components of an example sensitivity map: an input regridded SOS map, the map output by proposed network, and the conventional sensitivity map. The proposed sensitivity maps exhibit similar quality to the conventional maps, having removed artifacts and noise from the regridded SOS maps.
Figure 4, the multitasking reconstructions using different sensitivity maps can be seen. The image reconstructed by our proposed sensitivity maps shows high SNR and minimal artifacts, just as the one from conventional algorithms. Figure 5 shows difference maps comparing images reconstructed with conventional sensitivity maps to images reconstructed with either the input or output sensitivity maps to/from the proposed network (The magnitude of images were normalized to around 1).
Discussion and Conclusions
This work proposed a new deep learning algorithm to quickly generate high-quality coil sensitivity maps from non-Cartesian MR data. Sensitivity map quality and image reconstruction quality are similar to conventional sensitivity map estimation. Because the sensitivity map estimation requires only one forward pass through a trained network, which needs only 2.5s for 12 coils compared to 42s in conventional algorithm, the method shows promise for reducing the total image reconstruction time of non-Cartesian imaging. Besides, the outputting sensitivity maps of deep learning algorithm have much fewer singularities than the conventional outputs, which means the reconstruction image of the proposed methods can be better.Acknowledgements
This work was supported by NIH 1R01HL124649.References
1. Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med 1997;38(4):591-603.
2. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42(5):952-962.
3. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002;47(6):1202-1210.
4. Peng X, Perkins K, Clifford B, Sutton B, Liang Z-P. Deep-SENSE: Learning coil sensitivity functions for SENSE reconstruction using deep learning. ISMRM 2018;3528.
5. Christodoulou AG, Shaw JL, Nguyen C, Yang Q, Xie Y, Wang N, Li D. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nature Biomed Eng 2018;2(4):215-226.
6. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. MICCAI 2015;234-241.
7. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. IEEE-CVPR 2016;770-778.
8. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med 2014;71(3):990-1001.
9. Uecker M, Hohage T, Block KT, Frahm J. Image reconstruction by regularized nonlinear inversion—joint estimation of coil sensitivities and image content. Magn Reson Med 2008;60(3):674-682.
Figures
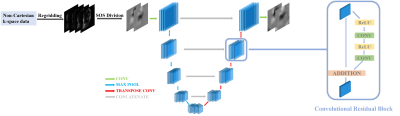
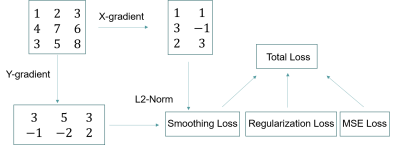
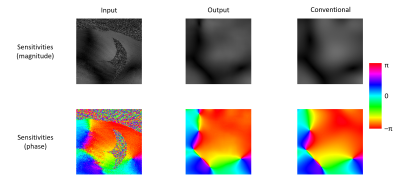
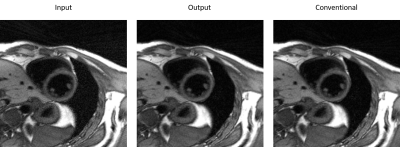
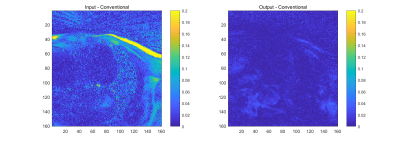
Figure 5. Left: Subtraction between the image reconstructed with the initial sensitivity maps and the conventional sensitivity maps.
Right: Subtraction between the image reconstructed with the output sensitivity maps and the conventional sensitivity maps.