4724
Automated Segmentation of Substantia Nigra in Neuromelanin-Sensitive Magnetic Resonance Imaging1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Cedars-Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Accurate segmentation of Substantia Nigra (SN) in Neuromelanin-Sensitive MRI (NM-MRI) is a prerequisite for efficient quantification and evaluation of severity of Parkinson disease. We present a fully automated algorithm for localization and segmentation of SN in NM-MRI. The localization algorithm uses a new specialized template matching model consisting of a resizable cardioid plane. The segmentation of SN is performed using freeform active contour segmentation model. The system is tested on 19 NM-MRI scans (10 healthy volunteers and 9 patients with Parkinson disease), acquired using 3T MRI system. The success rate for localization is 98.2%, whilst dice coefficient for segmentation reaches 0.89.
Introduction
Parkinson’s disease (PD) is a chronic degenerative neurological disorder characterized by depletion of catecholaminergic neurons in brainstem gray matter nuclei, such as the substantia nigra (SN) pars compacta [1]. The SN neurons contain a melanin pigment known as neuromelanin that can be readily detected by neuromelanin-sensitive MRI (NM-MRI) [2]. This biomarker has recently shown the potential to evaluate the severity of PD [3]. Thus, quantification of the depletion of neuromelanin may help tracking disease progression and inform the treatment efficacy. As a prerequisite to a quantitative analysis of neuromelanin in SN, we present an automated method to efficiently segment SN in MRI scans.Methods and Material
The methodology consists of an automated localization and segmentation phase as detailed below.
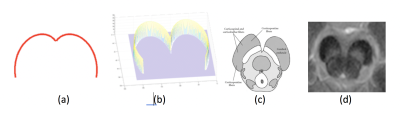
The localization step consists of identifying the general location of SN in each slice and is performed using a specialized template matching process. The template consists of a cardioid plane (created using mathematical equation for cardioid plane) which highly resembles to the general structure of Cerebral Peduncle (CP) within which SN lies. The CP is the part of midbrain and has consistent structure with rigid boundaries, and thus relatively easier to locate. The filter used a template consisting of a plane rotated cardioid curve (upper half) given by mathematical polar equation 𝑟 = 1 − 𝑠𝑖𝑔𝑛(𝜃) and cartesian equation (𝑥2 + 𝑦2 + 𝑎𝑥2)2 = 𝑎2(𝑥2 + 𝑦2). Before performing template matching, the Laplacian of Gaussian edge detection method is used to obtain edges in original greyscale slice (2D image). The localization algorithm then determines the correlation peak using template T and binary image, B (image with object edges) which gives an approximated center of CP in corresponding greyscale image. The correlation score (𝑆) for each generated template is calculated using Sum of Squared Differences (SSD) approach that uses Euclidian distance matrices for B and T.
The
SN segmentation is performed within specified
boundary of CP by firstly, specifying seeds points (pixels) that lie above 80th percentile
of the normalized intensity distribution of pixels that belong to the localized
CP region. The seed points initialize the active contour [5] that performs the
complete segmentation of SN in second step.
To validate the proposed method, a total of 19 NM-MRI scans (10 healthy, 9 patients with
PD) were acquired for this pilot study using our 3T system (mMR, Siemens
Healthineers, Erlangen, Germany) and an 8-channel head coil. The NM-MRI
technique used in this work is built on a magnetization-transfer prepared
gradient-echo sequence [4]. The imaging parameters included: number of slices
7, slice thickness 3 mm, in-plane resolution 0.35x0.35 mm2,
and, scan time 3 min 41 sec.
Results and Discussion
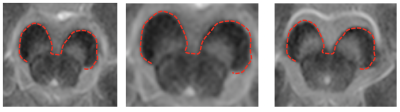
The system tested on 133 2D slices from 19 MRI scans by comparing the outcome with manually segmented CP and SN in all slices. The system successfully located SN by 98.2% and remained unsuccessful by 1.8%, i.e. on an average, 1.8% of SN was out of the localized region in all slices of 19 scans.
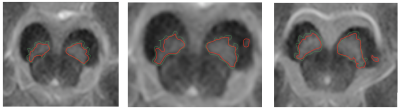
The outcome of the segmentation algorithm is compared with the manually segmented SN in all slices. The segmentation result is expressed in dice coefficient (DSC) as 0.89 using formula DSC=2TP/(2TP+FP+FN). The system performed well on both types of scans (i.e., healthy volunteers and patients with PD) with no significant difference of segmentation accuracy. A sample outcome of the localization and segmentation is shown in Figure 2 and Figure 3, respectively.
To proposed localization method is completely reproducible as templates of cardioid plane can be generated using a single line equation. Also, the templates generation is highly generalized, as the user can specify their own range of parameters in the cardioid equation to control size and shape of templates to obtain best possible results on their dataset.
By localizing SN in CP, the segmentation problem reduces to a binary pixel-classification problem where SN is bright region (foreground) on a dark region (background). The seed points in localized CP region work as hard constraints that assists active contour to efficiently initialize the segmentation of SN to achieve best possible results.
Conclusion
We presented an automated method to segment SN in NM-MRI. To localize the SN, a highly generalized and completely reproducible template-matching algorithm is introduced that doesn’t require real images to create templates. The SN segmentation within the localized region is performed using Active Contour. The overall system is robust, and the results achieved are sufficiently high.Acknowledgements
No acknowledgement found.References
[1] Greenfeld JG, Bosanquet FD. The brain-stem lesions in Parkinsonism. J Neurol Neurosurg Psychiatry 1953;16(4): 213–226.
[2] Sasaki M, Shibata E, Tohyama K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport 2006;17(11):1215– 1218.
[3] Schwarz ST, Xing Y, Tomar P, et al. In vivo assessment of brainstem depigmentation in Parkinson disease: potential as a severity marker for multicenter studies. Radiology 2017; 283: 789-798.
[4] Nakane T, Nihashi T, Kawai H, et al. Visualization of neuromelanin in the Substantia nigra and locus ceruleus at 1.5 T using a 3D-gradient echo sequence with magnetization transfer contrast. Magn Reson Med Sci 7: 205-210, 2008.
[5] Kass, Michael and Witkin, Andrew and Terzopoulos, Demetri, Snakes: Active contour models, International journal of computer vision, vol.1, num. 4, pages: 321-331, 1988.
Figures