4612
Water-fat separation in spiral readout acquisition for the liver using the convolutional neural network: an approach to reduce blurring artifacts1Department of Radiology, University of Yamanashi, Chuo, Japan, 2MRIsimulations Inc., Tokyo, Japan
Synopsis
Water/fat separation algorithm for two-echo spiral acquisition in the liver was developed using the convolutional neural network (CNN). The processing in the CNN was performed in the sinogram domain. A Bloch simulator was used to simulate the phase error in the k-space caused by the off-resonance components of background and fat. A volunteer study showed the successful water/fat separation using the proposed method without additional echoes of reference scans.
Introduction
A spiral readout is a promising approach to achieve fast acquisition in the liver. Generally, fat suppression techniques including spectrally-selective saturation pulses are required in spiral imaging because the acquisition with long readout time results in artifact caused by fat components1. However, it is challenging to perform uniform fat suppression in case of inhomogeneous B0 and B1 field. A chemical shift encoded imaging (CSI) is widely used to achieve uniform water-fat separation in clinical applications2,3. However, the CSI in spiral readout still has the blurring problem.
In this study, we developed a novel water-fat separation technique for spiral readout acquisition using the convolutional neural network (CNN) and the Bloch simulator to overcome the blurring problem.
Materials & Methods
Network architecture and training:
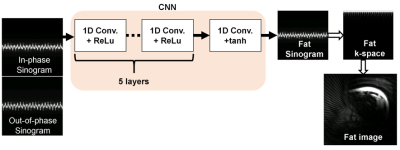
A simple CNN consists of six 1-dimensional (1D) convolution filters (kernel size = 5 with four channels) with rectified linear units (ReLU), followed by a 1D convolution filter (kernel size = 3 with four channels) with hyperbolic function was adopted as shown in Fig. 1. Sinograms of in-phase and out-of-phase k-spaces were used for the input of the network. Finally, the fat sinogram was predicted in the last layer. The water sinogram can be calculated by subtracting it from the in-phase sinogram. The optimization of the network was implemented using the stochastic gradient descent optimizer (learning rate = 0.0025, momentum = 10-6, momentum = 0.9) with 80 epochs. The mean square error was used for the loss function.
Training datasets:
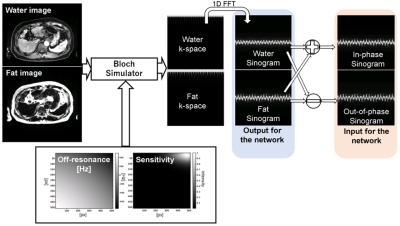
A procedure to obtain datasets for the training was summarized in Fig. 2. The datasets were generated from the water and fat MR images acquired using the two-point chemical shift imaging (LAVA-Flex, TR/TE1/TE2 = 3.9/1.2/2.4 ms, flip angle = 12°, matrix = 512×512, acquisition matrix = 320×224, FOV = 33-40 cm, slice thickness = 3 mm). The k-space datasets were calculated from the images using the Bloch simulator4, which enables realistic simulation of the blurring due to the fat and background off-resonance expressed as below equation.
$$$S(t) = \int \int (W e^{-j2 \pi (k_x(t)x + k_y(t)y)} + F e^{-j2 \pi (k_x(t)x + k_y(t)y)+\phi_f t}) e^{-2 \pi j \phi_0 t} dx dy,$$$
where S is the k-space signal, t is the sampling time, W and F are the water and fat images, kx and ky are the k-space coordinates along x and y, Φ0 and Φf are the background and fat off-resonance frequencies. The fixed T1 and T2 for the water (T1 = 1000 ms, T2 = 60 ms) and fat (T1 = 300 ms, T2 = 80 ms) components were used for the simulation. A spoiled gradient echo sequence (TR/TE1/TE2 = 20/2.2/3.3 ms, matrix = 320×320, FOV = 36 cm) with a 64-shot spiral readout (sampling point = 2048, dwell time = 2 microseconds) was used. The sinogram was derived by applying the Fourier transform to the k-space along the readout direction.
Experiment
We tested the proposed method with a volunteer. A 3-tesla clinical MRI scanner (GE Healthcare, Milwaukee, WI) with a 32-channel torso array and a three-echo spiral sequence (TR/TE1/TE2/TE3 = 20/2.2/3.3/4.4 ms, matrix = 320×320, FOV = 36 cm, slice thickness = 5 mm) with the trajectory adopted in the simulation was used. The acquisition was performed within one breath-hold to avoid image misregistration. The two echoes of in-phase (2.2 ms) and out-of-phase (3.3 ms) were used for the water/fat separation using the CNN. For the comparison of reconstruction techniques, water/fat separation using the three echoes (three-point chemical shift imaging: 3P-CSI)3 was performed. The reconstruction from the k-space to the image domain for the CNN and the 3P-CSI was implemented using the non-uniform Fourier transform (number of iteration = 15).
Results & Discussion
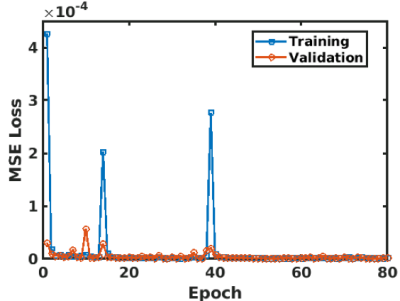
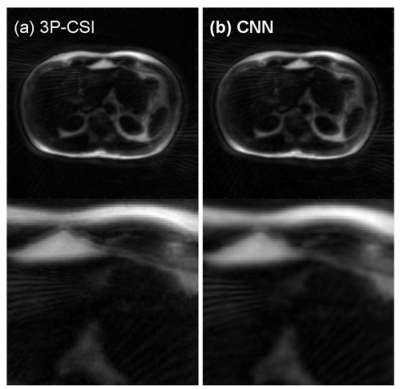
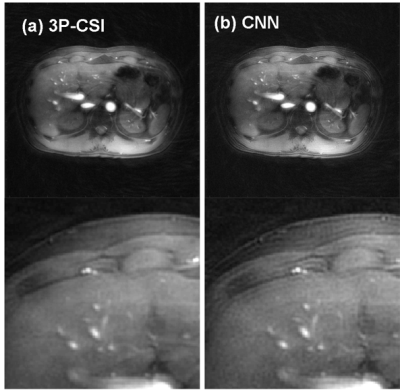
The training was successfully converged as shown in Fig. 3. The volunteer fat images calculated using the 3P-CSI and the CNN were shown in Fig. 4 (a) and (b). The water images using the 3P-CSI and the CNN were shown in Fig. 5 (a) and (b). These results implied that the water/fat separation with the CNN enabled sharper reconstruction while the 3P-CSI gave blurring due to the phase error during the sampling. There are currently some methods to correct the blurring for the spiral imaging1,5. Compare to the conventional methods, our approach has an advantage of the reconstruction without additional echoes or reference scans in addition to the simplicity of the processing.Conclusion
Water/fat separation approach for two-echo spiral imaging using the CNN was proposed. The processing was performed in the sinogram domain. The in-vivo study demonstrated the feasibility of our method.Acknowledgements
No acknowledgement found.References
[1] Moriguchi, Hisamoto, Jonathan S. Lewin, and Jeffrey L. Duerk. "Dixon techniques in spiral trajectories with off‐resonance correction: a new approach for fat signal suppression without spatial‐spectral RF pulses." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 50.5 (2003): 915-924.
[2] Dixon, W. Thomas. "Simple proton spectroscopic imaging." Radiology 153.1 (1984): 189-194.
[3] Glover, G. H., and E. Schneider. "Three‐point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction." Magnetic resonance in medicine 18.2 (1991): 371-383.
[4] Kose, Ryoichi, and Katsumi Kose. "BlochSolver: A GPU-optimized fast 3D MRI simulator for experimentally compatible pulse sequences." Journal of Magnetic Resonance 281 (2017): 51-65.
[5] Brodsky, Ethan K., et al. "Generalized k‐space decomposition with chemical shift correction for non‐Cartesian water‐fat imaging." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 59.5 (2008): 1151-1164.
Figures