4590
Reduced Noise Enhancement in Whole-Brain Multiband-RASER1Radiology, CMRR/University of Minnesota, Minneapolis, MN, United States
Synopsis
High acceleration is an effective tool to achieve whole brain coverage with acceptable total acquisition times. A limitation of parallel imaging is high noise amplification at high acceleration factors. In this work, an algorithm for parallel imaging of RASER in two dimensions implemented. The noise enhancement is theoretically derived and experimentally validated. Results show that even at high acceleration noise amplification remains low and high resolution whole brain images can be obtained with RASER.
Introduction
High acceleration is an effective tool to achieve whole brain coverage with acceptable total acquisition times. A limitation of parallel imaging is high noise amplification at high acceleration factors. In this work, an algorithm for parallel imaging of RASER (rapid acquisition by sequential excitation and refocusing) in two dimensions implemented. The noise enhancement is theoretically derived and experimentally validated. An example of a T2-weighted image of the human brain at 7 T is shown.Methods
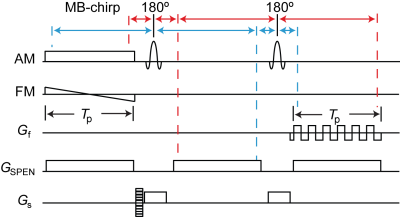
RASER is ultrafast imaging method using spatiotemporal encoding (SPEN) (Fig. 1)1, 2. The signal in the SPEN-dimension is sequentially excited by a chirp-pulse and in same order recorded during an echo readout train. The signal is localized as result of signal dephasing in the periphery of the quadratic phase profile generated by frequency-sweep of the chirp-pulse. The vertex of the quadratic phase moves along the SPEN-dimension encoding an image profile. The signal in the SPEN-dimension is, hence, acquired in the image domain. Parallel imaging in the SPEN-dimension was achieved by using multi-band excitation. The phase-encoded slab-selective dimension is undersampled for acceleration.
All experiments were performed on a 7 T scanner (Siemens, Erlangen, Germany) with Nova 8Tx 32Rx head coil. Full reference scans with two to four bands were acquired of a spherical phantom filled with silicone oil. Other imaging parameters were spatial resolution (2 mm)3, acquired matrix size: 200 x # bands·24 x 64. TE = 75 ms, TR = 800 ms. Acceleration along the SPEN-dimension was simulated by superimposing excitation bands. For the subject data, a full reference scan with short TR (800 ms) was acquired in addition to the T2-weighted accelerated scan with long TR (2 s). Imaging parameters are spatial resolution (1.25 mm)3, TE = 70 ms, multi-band 4, acceleration in the phase-encoded slab-selective dimension 4.
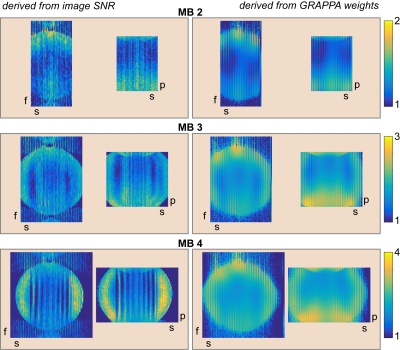
Images were reconstructed using GRAPPA according to the flow diagram in Fig. 2. The pseudo multiple replica method3 was used to compute g-factors from image SNR. G-factors were also theoretically derived from GRAPPA-weights.
Theory
To perform GRAPPA in the SPEN-dimension the signal, which is acquired in image space I, has to be Fourier-transformed to generate k-space data s:
$$s^{acc}_{\mu}=\sum_{\nu=1}^{N_{SPEN}}\tilde{I}_\nu\exp\left\{-\frac{2\pi i}{N_{SPEN}}\mu\nu\right\}$$[1]
where $$$\tilde{I}_\nu=\sum_{b=1}^{M_B}I_{B\nu}$$$ represents the superimposed signals from multi-band excitation. Considering the noise n during acquisition one obtains
$$s^{acc}_{\mu}+n'^{acc}_{\mu}=\sum_{\nu=1}^{N_{SPEN}}\tilde{I}_\nu\exp\left\{-\frac{2\pi i}{N_{SPEN}}\mu\nu\right\}+\sum_{\nu=1}^{N_{SPEN}}n^{acc}_\nu\exp\left\{-\frac{2\pi i}{N_{SPEN}}\mu\nu\right\}$$[2]
Assuming the noise is random and uncorrelated in respect to the frequency-encoded, SPEN- and phase-encoded slab-selective dimension the noise variance of the k-space signal is
$${\sigma^{2}}\left({n'^{acc}_{c}}\right)=N_{SPEN}{\sigma^{2}}\left({n^{acc}_{c}}\right)$$[3]
For the reference scan the noise propagation is
$$s^{full}_{\mu}+n'^{full}_{\mu}=\sum_{\nu=1}^{M_{B}N_{SPEN}}I_\nu\exp\left\{-\frac{2\pi i}{M_{B}N_{SPEN}}\mu\nu\right\}+\sum_{\nu=1}^{M_{B}N_{SPEN}}n^{full}_\nu\exp\left\{-\frac{2\pi i}{M_{B}N_{SPEN}}\mu\nu\right\}$$[4]
Hence, the noise variance in the reference scan is
$${\sigma^{2}}\left({n'^{full}_{c}}\right)=N_{SPEN}{\sigma^{2}}\left({n^{full}_{c}}\right)$$ [5]
Hence, the noise ratio in k-space before applying GRAPPA-reconstruction is
$$\frac{{\sigma^{2}}\left({n'^{acc}_{c}}\right)}{{\sigma^{2}}\left({n'^{full}_{c}}\right)}=\frac{1}{M_{B}}\frac{{\sigma^{2}}\left({n^{acc}_{c}}\right)}{{\sigma^{2}}\left({n^{full}_{c}}\right)}$$[6]
Using the definition of the g-factor in reference4 one obtains for multi-band acceleration
$$\frac{SNR_{full}}{SNR_{acc}}=\frac{1}{M_B}g;g^2=\frac{1}{N_z}\sum_{z=1}^{N_z}g^2_z$$[7]
where gz is the g-factor for each phase-encoding step in the slab-selective dimension.
Results
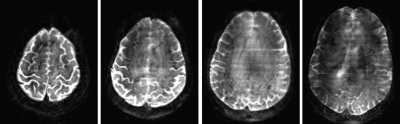
Results obtained from the phantom data (Fig. 3) demonstrate that the g-factor maps derived from image SNR using equ. (7) and from GRAPPA-weights4 are similar. As expected the g-factor increases with the number of bands. In contrast the noise amplification as described by equ. (7) remains low even with a high number of bands. Fig. 4 shows an example of a whole brain T2-weighted RASER image of a human brain acquired with accelerated SPEN as well as the phase-encoded slab-selective dimension and reconstructed with the algorithm in Fig. 2.Conclusion
High acceleration can be achieved with RASER by accelerating both the SPEN and the phase-encoded slab-selective dimension. Even at a high number of bands noise amplification remains low. Hence, whole brain coverage with RASER in high-resolution imaging is feasible.Acknowledgements
Financial support by the NIH-grants BTRC P41 EB015894, P30 ICC NS076408, S10 RR026783 and the Keck Foundation is acknowledged.References
1. Chamberlain R, Park JY, Corum C, Yacoub E, Ugurbil K, Jack CR, Garwood M. RASER: A new ultrafast magnetic resonance imaging method. Magn Res Med 2007;58(4):794-799.
2. Goerke U, Garwood M, Ugurbil K. Functional magnetic resonance imaging using RASER. NeuroImage 2011;54(1):350-360.
3. Robson PM, Grant AK, Madhuranthakam AJ, Lattanzi R, Sodickson DK, McKenzie CA. Comprehensive quantification of signal-to-noise ratio and g-factor for image-based and k-space-based parallel imaging reconstructions. Magn Res Med 2008;60(4):895-907.
4. Breuer FA, Kannengiesser SAR, Blaimer M, Seiberlich N, Jakob PM, Griswold MA. General Formulation for Quantitative G-factor Calculation in GRAPPA Reconstructions. Magn Res Med 2009;62(3):739-746.
Figures