4559
T1 measurement in short-T2 material with suppressed long-T2 component using an IR-UTE multishot sequence1Aix-Marseille Univ. CRMBM UMR 7339, Marseille, France, 2Université de Strasbourg, CNRS, ICube, FMTS, Strasbourg, France, 3Institut Mines Télécom Atlantique, INSERM, LaTIM, Brest, France
Synopsis
T1 quantification of short-T2 species is challenging due to the uncommon behavior of the signal decay and magnetization tilting during excitations in conventional sequences. UTE sequences can therefore be considered with refined magnetization evolution models using the Bloch equations. In voxels comprising a mix of long and short-T2 components (e.g. myelin and water in the normal appearing white matter), an appropriate long-T2 suppression scheme is mandatory. In this work, we propose an analytical model to quantify the T1 of a short-relaxing component in an accelerated Inversion-Recovery UTE in vitro, and within long-T2 suppression condition.
Introduction
T1 measurement of short-T2 ($$$T_2<1$$$ ms) species in mixed tissues composed of long ($$$T_2>10$$$ ms) and short-T2 components is challenging as it requires to cancel the long-T2 signal to properly discriminate the component of interest. The Inversion-Recovery-UTE method was previously employed1, assuming a saturation of a single short-T2 component in the cortical bone and in the myelin.
In this work, we propose to use an accelerated IR-UTE sequence using a multispoke acquisition scheme (IR-UTE-MS)2-4 in a single TR. The method is presented as a proof of concept to quantify the T1 value of cortical bone and Lego brick within a suppressed long-T2 condition.
Method
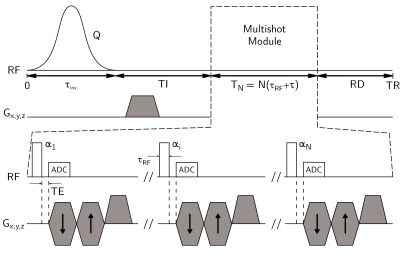
Figure 1 shows the IR-UTE-MS pulse sequence used in experiment.
In IR-UTE-MS, the short-T2 magnetization can be expressed as2,4:
$$M_{z,k}^-=M_{z,1}^{-}E_\tau^{k-1}\displaystyle\prod_{p=1}^{k-1}\cos(\alpha_p)+M_0(1-E_\tau)\sum_{i=1}^{k-1}E_\tau^{i-1}A_i$$
with $$$M_0$$$ magnetization at thermal equilibrium, $$$A_1=1$$$, and $$$A_i=\prod_{p=k+1-i}^{k-1}\cos(\alpha_p)$$$ otherwise, $$$M_{z,k}^{-}$$$ longitudinal magnetization prior to the k-th pulse, and:
$$M_{z,1}^{-}=M_0\frac{(1-E_I)+E_I(1-E_{RD}E_\tau)Q+QE_IE_{RD}(1-E_\tau)\displaystyle\sum_{i=1}^{N-1}E_\tau^{i}\displaystyle\prod_{p=N+1-i}^{N}\cos(\alpha_p)}{1-QE_IE_{RD}E_\tau^{N}\displaystyle\prod_{p=1}^{N}\cos(\alpha_p)}$$
with Q inversion efficiency, $$$\{E_I,E_\tau,E_{RD}\}=\{e^{-TI/T_1},e^{-\tau/T_1},e^{-RD/T_1}\}$$$, T1/T2 longitudinal and transverse relaxation times, RD resting delay before TR, and N the number of spokes per TR. Due to the averaging pattern of radial acquisitions3, we consider the following model for T1 and Q estimation4:
$$S^N(S_0,T_1,Q)=\frac{G}{N}\times\left|\sum_{k=1}^NM_{z,k}^-(T_1,Q)\sin(\alpha_k)\right|$$
where $$$G$$$ is a constant, and $$$S_0=G\times M_0$$$.
The IR-UTE sequence is a particular case of the IR-UTE-MS sequence (N=1). From previously defined equations, the longitudinal short-T2 magnetization before the excitation is reduced to:
$$M_{z}^-=M_0\frac{1-E_I+E_I(1-E_{RD}E_\tau)Q}{1-QE_IE_{RD}E_\tau\cos(\alpha)}$$
where $$$\alpha$$$ is the single excitation flip angle. This similarly yields the following model parametric quantification:
$$S^{1}(S_0,T_1,Q)=G\times|M_{z}^-(T_1,Q)\sin(\alpha)|$$
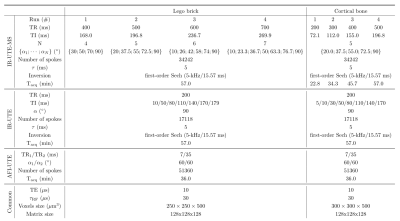
Two phantoms were constituted: one composed of a piece of Lego brick ($$$T_1\approx300$$$ ms at 2.48 T5), and one with a human humerus bone sample with removed tissues and bone marrow ($$$T_1\approx213$$$ ms at 3 T6). Both sample were soaked into a doped water (1.5%-agarose/0.5 mM Ni$$$^{2+}$$$; $$$T_1/T_2=1400/70$$$ ms), and scanned using a 7T preclinical scanner (Bruker BioSpec, Ettlingen, Germany) using a 72-mm Tx/Rx volume coil. Details of the respective IR-UTE-like protocols are given in Table 1:
- IR-UTE: to provide a ground truth for T1 and Q estimation in the short-T2 materials, multi-TI IR-UTE experiments were performed;
- IR-UTE-MS: the long-T2 signal was simulated using a configuration states framework7 in order to provide an accurate TI for signal cancellation, while accounting for transverse states contributions throughout the consecutive pulses (with RF pulses phases equal to $$$0^\circ$$$)4. 4 runs were performed for each sample with different optimized schemes (equal acquisition time for the Lego brick phantom, and same readout pattern for the cortical bone one). Flip angle series were chosen non-constant in order to lower potential signal variability effects throughout the consecutive pulses3;
- A B1 mapping sequence based of the Actual Flip Angle technique8,9 was adapted with a 3D-UTE readout module (AFI-UTE)6. The estimated B1 values were used to correct the flip angles during the fitting processes.
Mean signals from ROIs drawn in the short-T2 materials were used for parametric estimations, using models $$$S^N$$$ and $$$S^1$$$ for IR-UTE-MS and IR-UTE, respectively. Fit results are expressed as (fit value $$$\pm$$$ Cramér-Rao lower bound).
Results
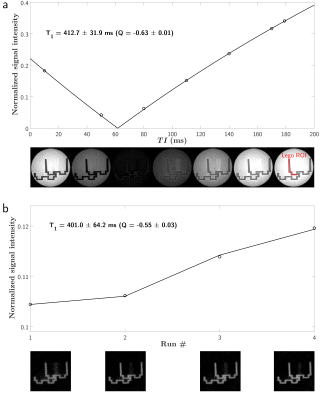
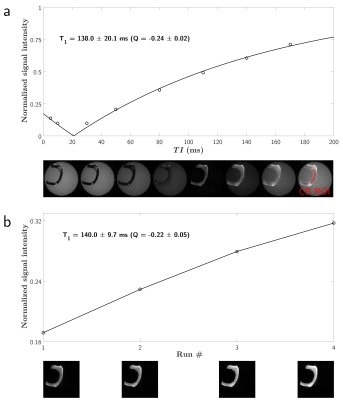
Figure 2 and 3 show experimental and fitted curves of the two different methods, as well as views of the respective sample. The AFI-UTE estimated a mean relative B1 of 0.97 in the Lego brick, and 0.96 in the bone sample. Estimated parameters amounted to $$$T_1=412.7\pm31.9$$$ ms and $$$Q=-0.63\pm0.01$$$ in IR-UTE versus $$$T_1=401.0\pm64.2$$$ ms and $$$Q=-0.55\pm0.03$$$ in IR-UTE-MS in the Lego brick. Similarly, these parameters were $$$T_1=138.0\pm20.1$$$ ms and $$$Q=-0.24\pm0.02$$$ in IR-UTE versus $$$T_1=140.0\pm9.7$$$ ms and $$$Q=-0.22\pm0.05$$$ in IR-UTE-MS in the cortical bone. Models allowed excellent fitting ($$$R^2_{adj}>0.99$$$), with rather close estimated parameters between IR-UTE and IR-UTE-MS.Discussion and conclusion
The IR-UTE-MS method allowed for T1 quantification in an appropriate long-T2 suppression condition.
Parameters sensitivity of the model has yet to be extensively explored, but nonetheless provided interesting results in vitro. Flip angles were also considered to be short enough to prevent from relaxation-excitation effects10, and excitation variability was assimilated to a simple B1 inhomogeneity6. Further work may take into account actual irreversible excitation T2-effects by substituting the cos and sin terms with mapping functions10 in the models.
Overall, IR-UTE-MS is a promising method for the exploration of semi-solid structures requiring long-T2 suppression such as myelin, and might be further used for proton density quantifications.
Acknowledgements
The authors thank Professor Jean-Luc Kahn and Noël Zuckschwert from the Institute of Anatomy of Strasbourg for providing the bone sample thanks to a body donation to medical science.References
1. Du J, Sheth V, He Q, Carl M, Chen J, CoreyBloom J, Bydder GM. Measurement of T1 of the Ultrashort T2* Components in White Matter of the Brain at 3T. PLoS ONE 2014;9:e103296
2. Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magnetic Resonance in Medicine 2016;76:577–582
3. Li C, Magland JF, Zhao X, Seifert AC, Wehrli FW. Selective in vivo bone imaging with long-T2 suppressed PETRA MRI. Magnetic Resonance in Medicine 2017;77:989–997
4. Soustelle L, Lamy J, Rousseau F, Armspach J, Loureiro de Sousa P. Inversion-Recovery UTE multispoke sequence: comparison of two excitation schemes. in Proceedings of the 26th Annual Meeting of ISMRM, Paris, France, 2018. p. 4071
5. Codd S, Mallett M, Halse M, Strange J, Vennart W, Doorn T. A Three-Dimensional NMR Imaging Scheme Utilizing Doubly Resonant Gradient Coils. Journal of Magnetic Resonance, Series B 1996;113:214–221
6. Han M, Larson PE, Krug R. Actual Flip Angle Imaging to Improve T1 Measurement for Short T2 Tissues. in Proceedings of the 23th Annual Meeting of ISMRM, 2015, p. 501
7. Weigel M. Extended phase graphs: Dephasing, RF pulses, and echoes - pure and simple. Journal of Magnetic Resonance Imaging 2015;41:266–295
8. Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: A method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magnetic Resonance in Medicine 2007;57:192–200
9. Nehrke K. On the steady-state properties of actual flip angle imaging (AFI). Magnetic Resonance in Medicine 2009;61:84–92
10. Sussman MS, Pauly JM, Wright GA. Design of practical T2-selective RF excitation (TELEX) pulses. Magnetic Resonance in Medicine 1998;40:890–899
Figures