4546
A robust pulse sequence for simultaneous diffusion MRI and MR elastography (diffusion-MRE)1Department of Radiological Sciences, Graduate School of Human Health Sciences, Tokyo Metropolitan University, Tokyo, Japan, 2Office of Radiation Technology, Keio University Hospital, Tokyo, Japan, 3Health Research Institute, National Institute of Advanced Industrial Science and Technology, Ibaraki, Japan, 4Department of Mechanical Engineering, Tokyo Denki University, Tokyo, Japan
Synopsis
Diffusion-magnetic resonance elastography (dMRE) can acquire diffusion and mechanical properties simultaneously. However, intravoxel phase dispersion (IVPD) interferes with the calculation of the apparent diffusion coefficient (ADC). This study presents an approach to dMRE that reduces the influence of IVPD by introducing a new pulse sequence. The ADC and stiffness, obtained using the existing and proposed dMRE techniques, were compared with spin-echo (SE)-diffusion and SE-MRE, for a phantom. In existing dMRE technique, the ADC was changed by IVPD but that of proposed dMRE technique was unchanged. The results demonstrate that our dMRE technique is a robust method for addressing the IVPD.
INTRODUCTION
MR elastography (MRE) and diffusion magnetic resonance imaging (dMRI) provide quantitative information about mechanical properties (such as stiffness) and diffusion of water molecules. Combining MRE with dMRI has the potential for more precisely diagnosing the stages of prostate cancer and Alzheimer's Disease (AD) and evaluating tissue anisotropy1-3.
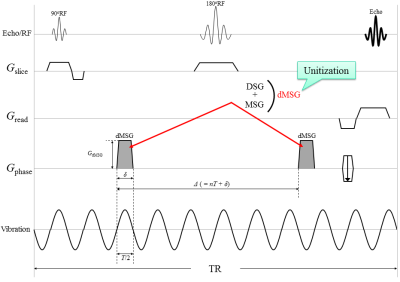
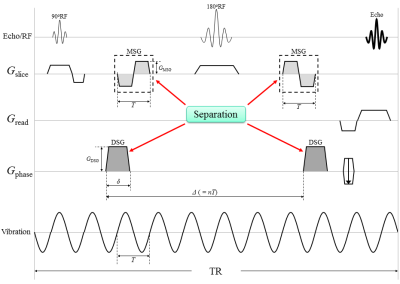
MRE uses bipolar gradient lobes in a motion-sensitizing gradient (MSG) to detect tissue displacements attributable to mechanical vibrations are encoded as phase shifts in a phase image; dMRI also uses bipolar gradient lobes in a diffusion-sensitizing gradient (DSG) to detect diffusion of water molecules are encoded as signal losses in a magnitude image. Yin et al.4 introduced diffusion-MRE (dMRE) technique, to simultaneously acquire MRE and dMRI. However, their technique exhibits a weak point in its susceptibility to the influence of intravoxel phase dispersion (IVPD)5, which causes signal losses on the magnitude image, because of using the bipolar gradients as both diffusion-sensitizing and motion-sensitizing (dMSG).
We designed a new pulse sequence that sets the MSG and DSG separately to reduce the effects of IVPD. Our new approach allows adjustments of the duration between DSG pairs, thus canceling out the phase shift from the DSG pairs.This study names this new method a “separate-gradient dMRE” (SG-dMRE) and the previous method a “unit-gradient dMRE” (UG-dMRE). We evaluated the accuracy of these techniques by working with a phantom.
METHODS
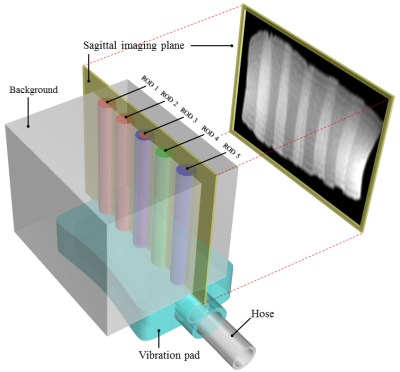
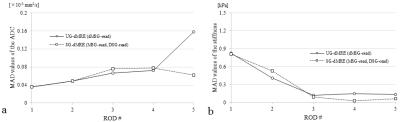
All experiments were performed using a 2.0 T animal MR scanner system (BioSpec 20/30, Bruker Corporation, Karlsruhe, Germany) and a 140 mm ID birdcage coil. UG-dMRE and SG-dMRE were performed using a phantom comprising five rods with different elasticities at 60 Hz vibration in one sagittal slice (Fig. 1). The sequences were compared under the following conditions: (1) mechanical vibrations with the same amplitude; (2) same total acquisition time; (3) less than 50 mT/m gradient strength; and (4) gradient duration obtaining a maximum detection sensitivity of 60 Hz vibration (Fig. 2 and 3). This study performed four time-step acquisitions to obtain images at four different b-values (0 s/mm2, 300 s/mm2, 700 s/mm2, and 1000 s/mm2). Additional acquisition parameters were as follows: 92×48 acquisition matrix, 1 number of averages, 90° flip angle, 130 mm2 field of view, 3 mm slice thickness, HF (readout) dMSG/DSG/MSG direction, 8.3 or 10 ms dMSG or DSG lobe duration (δ), 91.7 or 66.7 diffusion time (Δ) in UG-dMRE or SG-dMRE, 1 or 2 number of dMSG or MSG pairs, 700 ms repetition time, and 4.5 min total acquisition time (4 phase offsets). The measures of ADC and shear modulus, obtained by using both dMRE techniques, were compared with conventional spin-echo (SE)-diffusion and SE-MRE. Then, we evaluated those differences by using the mean of absolute differences (MAD) in the regions of interest (ROIs) for each of the five rods within the phantom.RESULTS
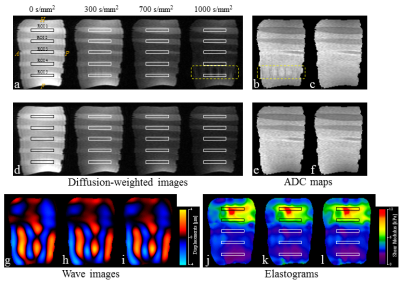
Figure 4 shows the diffusion-weighted images in each b-value (0–1000 s/mm2), wave images and elastograms in the results. The results of the MAD of the stiffness from both dMRE techniques showed almost no difference (Fig. 5a). In contrast, the value of the ADC MAD (MAD ≒ 0.16×10−3 mm2/s), obtained in the soft region within the phantom with UG-dMRE technique, was large (Fig. 5b).DISCUSSION
The signal loss from IVPD depends on various experimental conditions such as wave amplitude and frequency, gradient strength and duration, tissue stiffness, and voxel size. In our data, IVPD was caused near ROD 5 in UG-dMRE in the 1000 s/mm2 b-value, that is strong gradient. In addition, the wave displacement in ROD 5 was greater than for the other rods, as Fig. 4g–i shows. Thus, the signal losses from IVPD, identified in this study, may be attributed to greater values of dMSG and wave displacement. SG-dMRE has some advantages in addition to reducing the effects of IVPD compared with UG-dMRE. We can set arbitrary diffusion-weighted and vibration-sensitized effects and direction in SG-dMRE, although UG-dMRE could detect these qualities only in the same direction. The dMSG lobe duration (δ) becomes short as the vibration frequency is increased in UG-dMRE since the duration of dMSG must be matched to a vibration cycle to efficiently detect the vibration. Therefore, UG-dMRE can lead to a weak diffusion-weighted effect in the case of a high vibration frequency because of the limitation of the available gradient strength. On the other hand, SG-dMRE can attain a strong diffusion-weighted effect without depending on vibration frequency because the DSG lobe duration (δ) can be set to be arbitrary.CONCLUSION
Our results demonstrate that SG-dMRE sequence, a robust technique for overcoming the effects of IVPD, enables the simultaneous acquisition of dMRI and MRE information.Acknowledgements
No acknowledgement found.References
1. Hoyt K, Castaneda B, Zhang M, et al. Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomark 2008;4(4–5):213–225.
2. Gerischer LM, Fehlner A, Kobe T, et al. Combining viscoelasticity, diffusivity and volume of the hippocampus for the diagnosis of Alzheimer's disease based on magnetic resonance imaging. Neuroimage Clin 2018;18:485–493.
3. Qin EC, Sinkus R, Geng G, et al. Combining MR elastography and diffusion tensor imaging for the assessment of anisotropic mechanical properties: a phantom study. J Magn Reson Imaging 2013;37(1):217–226.
4. Yin Z, Magin RL, Klatt D. Simultaneous MR elastography and diffusion acquisitions: diffusion-MRE (dMRE). Magn Reson Med 2014;71(5):1682–1688.
5. Glaser KJ, Felmlee JP, Manduca A, et al. Shear stiffness estimation using intravoxel phase dispersion in magnetic resonance elastography. Magn Reson Med 2003;50(6):1256–1265.
Figures