4474
Respiratory Motion Signals Extracted from 3D Image-Based Navigation and from PCA of SI-Projections: Initial Findings in Whole-Heart Imaging using a Free-Running Framework1Department of Radiology, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 3Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 4LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
In continuously acquired whole-heart coronary MRA, it is not fully understood how unitless respiratory self-gating signals relate to actual respiratory displacement and drift. Therefore, self-gating signals extracted from principal component analyses of 1D projections oriented in the superior-inferior (SI) direction were compared to image-based navigators. Whole-heart data from continuous uninterrupted 3D radial bSSFP acquisitions were used to reconstruct time series of 3D sub-images with a temporal resolution of 0.6 seconds. Preliminary findings suggest that the SI-directed motion obtained from these sub-images is better described by respiratory self-gating signals created from three principal components rather than from one principal component alone.
Introduction
Recent Coronary Magnetic Resonance Angiography (CMRA) sequences that capture both cardiac anatomy and function in single multipurpose acquisitions1,2 show promise for improving the time-efficiency and ease-of-use of cardiac MR. Encouraging results have also been demonstrated in terms of image quality1–4 and agreement with 2D cine imaging in the assessment of cardiac function1–3. Image reconstruction strategies for these acquisitions typically sort the acquired data into several respiratory positions according to a respiratory self-gating signal.1,3,4 In Pang et al.1, respiratory motion was extracted using Principal Component Analysis (PCA) applied to 1D projection images in the Superior-Inferior (SI) direction. Specifically, a Principal Component (PC) with high energy in the range of respiratory frequencies of 0.1-0.5 Hz was considered to represent respiration. Although such a component is likely to capture the oscillatory behavior of respiration, an open question is how it accounts for respiratory drift, which is common in free-breathing CMRA5,6. Potentially, several PCs may be required for a more complete description of respiratory motion that includes drift. Image-based navigation7–11 may allow for the depiction of drift, but it has, to the best of our knowledge, not yet been investigated in the context of continuous uninterrupted non-ECG-gated free-breathing CMRA. Therefore, we aim to reconstruct 3D sub-images with high temporal resolution from the imaging data of such acquisitions in order to relate the SI-motion in these to self-gating using one or more PCs.Methods
Datasets from N=4 healthy subjects (2M, 32±4y) who provided written informed consent were included in this IRB-approved study. Acquisitions were performed on a 1.5T clinical MRI scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) using a prototype continuous free-breathing whole-heart bSSFP sequence (Fig.1) employing LIBRE water excitation pulses12 and 3D radial sampling13. For each subject, respiratory motion was estimated using both image-based navigation and respiratory self-gating (Fig.2). For image-based navigation, time series of 1149 3D sub-images were reconstructed, providing a temporal resolution of 0.60 seconds along the 11:33 minute acquisitions. Each sub-image was reconstructed from the 18 central k-space samples of 110 spokes, after low-pass filtering and Voronoi density compensation14. Subsequently, the SI-directed motion over time was estimated by performing a rigid translational co-registration of all the sub-images to an end-expiratory reference position15. In addition, two different PCA-based self-gating signals were extracted from the SI-projections (temporal resolution of 0.12 seconds): one containing only the PC with the highest energy in the respiratory range (1PC)4 and a second one (3PC) that also contained the two PCs that explained the most variance in the data, hypothesizing that they might capture respiratory drift. To relate the self-gating signals to the SI-displacement from the 3D sub-images (interpolated to matching temporal resolution), the Power Spectral Densities (PSDs) of the different signals were compared and Pearson’s correlation coefficients computed.Results
3D sub-images resolving respiratory motion were successfully reconstructed from the continuously acquired CMRA imaging data. The estimated motion along the SI-direction from the sub-image co-registration as well as the 1PC and 3PC self-gating signals showed high variability among the four subjects (Fig.3). The sub-image displacement curves were similar to the signals using 3PC in terms of shape and trend, while a different trend typically was seen in 1PC. The PSDs were highly consistent in the range of respiratory frequencies (Fig.4). On average, the correlation coefficients were 0.43± 0.056 for 1PC and 0.90±0.022 for 3PC (Fig.4). In Figure 5, the SI-motion signal from image-based navigation and corresponding end-expiratory sub-images consistently suggest respiratory drift over time.Discussion
The observation that the respiratory self-gating signals corresponding to 3PCs, rather than to 1PC, showed a closer correlation with the SI-motion signals extracted from co-registration of 3D sub-images may suggest that using 1PC for self-gating is an over-simplification in some cases. In three subjects, the 1PC signals did not account for cranial drift seen in the sub-images (Fig.3). Ideally, there would be a one-to-one correspondence between the sub-image displacement and the self-gating signals’ amplitudes in Figure 4. However, this might not be easily achieved due to the hysteresis of respiratory motion16, system imperfections and residual cardiac motion affecting the co-registration. In order to understand which type of respiratory self-gating signals best describes the respiratory-induced displacement of the heart, the resulting image quality of respiratory motion-resolved or motion-corrected reconstructions remains to be compared. Future work will also incorporate spatio-temporal correlations for improved sub-image quality, optimization of the image registration and exploiting 3D motion for binning.Conclusion
The respiratory self-gating signals created from three rather than from one principal component showed a better correlation with SI-motion signals obtained with image-based navigation. This suggests that respiratory self-gating using 3D sub-images or multi-component PCA should be further explored to guide reconstruction of continuous free-breathing whole-heart acquisitions.Acknowledgements
No acknowledgement found.References
1. J. Pang et al., “ECG and navigator-free four-dimensional whole-heart coronary MRA for simultaneous visualization of cardiac anatomy and function,” Magn. Reson. Med., vol. 72, no. 5, pp. 1208–1217, 2014.
2. S. Coppo et al., “Free-running 4D whole-heart self-navigated golden angle MRI: Initial results,” Magn. Reson. Med., vol. 74, no. 5, pp. 1306–1316, 2015.
3. L. Feng et al., “5D whole-heart sparse MRI,” Magn. Reson. Med., vol. 79, no. 2, pp. 826–838, 2018.
4. L. Di Sopra, D. Piccini, S. Coppo, J. A. M. Bastiaansen, M. Stuber, and J. Yerly, “Motion-Resolved 5D Imaging of the Heart: Time to Get Rid of the ECG?,” in Proceedings of the Annual ISMRM Meeting, 2017.
5. S. Kato et al., “Assessment of coronary artery disease using magnetic resonance coronary angiography: A national multicenter trial,” J. Am. Coll. Cardiol., vol. 56, no. 12, pp. 983–991, 2010.
6. M. Stuber, P. G. Danias, R. M. Botnar, D. K. Sodickson, K. V. Kissinger, and W. J. Manning, “Superiority of prone position in free-breathing 3D coronary MRA in patients with coronary disease,” J. Magn. Reson. Imaging, vol. 13, no. 2, pp. 185–191, 2001.
7. M. Henningsson, P. Koken, C. Stehning, R. Razavi, C. Prieto, and R. M. Botnar, “Whole-heart coronary MR angiography with 2D self-navigated image reconstruction,” Magn. Reson. Med., vol. 67, no. 2, pp. 437–445, 2012.
8. M. Henningsson, J. Smink, R. Razavi, and R. M. Botnar, “Prospective respiratory motion correction for coronary MR angiography using a 2D image navigator,” Magn. Reson. Med., vol. 69, no. 2, pp. 486–494, 2013.
9. H. H. Wu, P. T. Gurney, B. S. Hu, D. G. Nishimura, and M. V. McConnell, “Free-breathing multiphase whole-heart coronary MR angiography using image-based navigators and three-dimensional cones imaging,” Magn. Reson. Med., vol. 69, no. 4, pp. 1083–1093, 2013.
10. M. H. Moghari et al., “Three-dimensional heart locator for whole-heart coronary magnetic resonance angiography,” Magn. Reson. Med., vol. 71, no. 6, pp. 2118–2126, 2014.
11. J. Luo et al., “Nonrigid Motion Correction With 3D Image-Based Navigators for Coronary MR Angiography,” Magn. Reson. Med., vol. 77, no. 5, pp. 1884–1893, 2017.
12. J. A. M. Bastiaansen and M. Stuber, “Flexible water excitation for fat-free MRI at 3T using lipid insensitive binomial off-resonant RF excitation (LIBRE) pulses,” Magn. Reson. Med., vol. 00, no. May, 2017.
13. D. Piccini, A. Littmann, S. Nielles-Vallespin, and M. O. Zenge, “Spiral phyllotaxis: The natural way to construct a 3D radial trajectory in MRI,” Magn. Reson. Med., vol. 66, no. 4, pp. 1049–1056, 2011.
14. V. Rasche, R. Proksa, R. Sinkus, P. Börnert, and H. Eggers, “Resampling of data between arbitrary grids using convolution interpolation,” IEEE Trans. Med. Imaging, vol. 18, no. 5, pp. 385–392, 1999.
15. D. Piccini, G. Bonanno, G. Ginami, A. Littmann, M. O. Zenge, and M. Stuber, “Is there an optimal respiratory reference position for self-navigated whole-heart coronary MR angiography?,” J. Magn. Reson. Imaging, vol. 43, no. 2, pp. 426–433, 2016.
16. K. Nehrke, P. Börnert, D. Manke, and J. C. Böck, “Free-breathing Cardiac MR Imaging: Study of Implications of Respiratory Motion—Initial Results,” Radiology, vol. 220, no. 3, pp. 810–815, 2001.
Figures
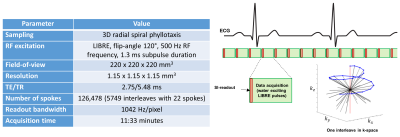
Figure 1. Sequence parameters and overview of the continuous uninterrupted free-breathing bSSFP acquisition
All acquisitions were performed using a continuous free-breathing bSSFP sequence with uninterrupted steady state. To achieve fat suppression, Lipid Insensitive Binomial Off-Resonant RF Excitation (LIBRE) pulses were used for water excitation. The k-space sampling followed a 3D radial spiral phyllotaxis sampling scheme designed to enable self-gating by consistently acquiring a readout in the SI-direction every 22nd line.
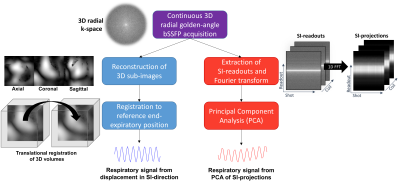
Figure 2. Overview of two different ways to extract respiratory motion
Two fundamentally different ways of extracting respiratory motion in the Superior-Inferior (SI) direction were utilized in this study. The first method was to perform a rigid translational co-registration of a time-series of reconstructed 3D sub-images to a manually chosen end-expiratory reference position (blue). The second method used Principal Component Analysis (PCA) of the SI-projections (red).
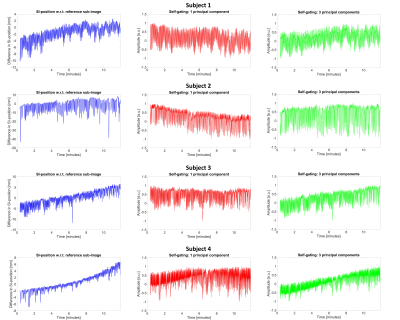
Figure 3. Respiratory motion signals
Respiratory motion signals from all four subjects originating from the difference in SI-position of the co-registered sub-images (blue) and principal component analysis of SI-projections (red = 1 principal component, green = 3 principal components, normalized to [-1, 1] after the signal extraction for display purposes).
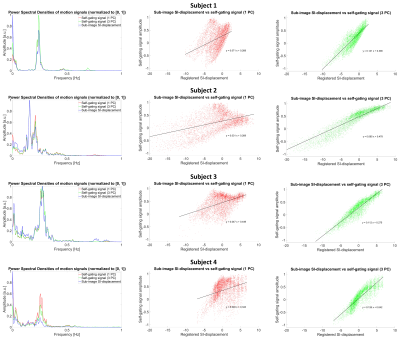
Figure 4. Power spectral densities and scatter plots showing the sub-image SI-displacement versus the self-gating signals’ amplitudes
The first column shows the PSDs of the motion signals from the sub-images, the one Principal Component (PC) with the highest energy in the range of respiratory frequencies (1PC) and the combination of the former and the two PCs that explained the most variance in the data (3PC). The second and third columns contain scatter plots depicting how the 1PC and 3PC self-gating signals’ normalized amplitudes vary as a function of the Superior-Inferior (SI) displacement extracted from the time series of 3D sub-images.
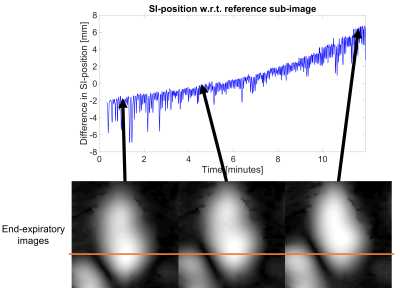
Figure 5. Example of subject that presents respiratory drift in cranial direction
Example showing how the end-expiratory level changes over the acquisition time in subject 4. Note e.g. how the position of the liver drifts.