4259
Beta-amyloid specific in vivo MR imaging with chalcone conjugated gadolinium complex.1Medical and bioengineering, Kyungpook national university, Daegu, Korea, Republic of, 2Molecular Medicine & BK21 Plus KNU Biomedical Convergence Program, Kyungpook national university, Daegu, Korea, Republic of, 3Institute of Biomedical Engineering Research, Kyungpook national university, Daegu, Korea, Republic of, 4Laboratory Animal Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea, Republic of, 5Radiology, Kyungpook national university, Daegu, Korea, Republic of
Synopsis
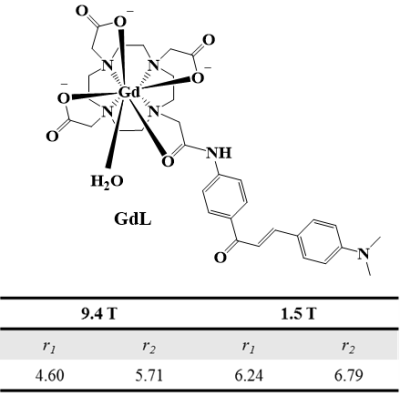
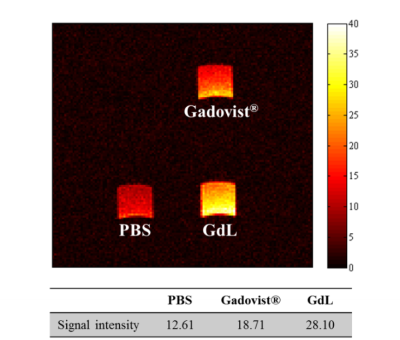
A new gadolinium complex conjugated with chalcone derivative (GdL) shows beta-amyloid specific enhancement in vivo MR imaging. The enhanced region in MR image matched well with fluorescence imaging of beta-amyloid plaque, which was stained by thioflavin S. The difference of signal enhancement between GdL and Gadovist in beta-amyloid oligomer supported that GdL has specificity on beta-amyloid. In addition, GdL shows considerable relaxivities even at high field 9.4 T (r1 = 4.60, r2 = 5.71 mM-1s-1) and comparable kinetic stability compared with commercial MRI agents.
Introduction
Detection of β-amyloid (Aβ) plaques with noninvasive method is critical in early diagnosis and guiding treatment of Alzheimer’s disease (AD). Magnetic resonance imaging (MRI) is a powerful diagnostic tool with high resolution, however, only large and mature senile β-amyloid (Aβ) plaques could be visualized for late stage AD patients and even those required a high magnetic field and very long scan time. Therefore, a probe that could specifically bind to plaques and visualized them in MRI might make a feasible early detection tool for AD. Recently, chalcone derivatives emerged as Aβ plaques imaging probe with high binding affinity for Aβ plaques. In this study, we prepared a new gadolinium complex conjugated with chalcone derivative (GdL) for Aβ imaging and MR study was carried out to confirm targeting ability.Materials and Methods
The relaxivity study was carried out at 1.5 T MR unit (64MHz, GE Healthcare) and 9.4 T MR unit (400MHz, Bruker). T1 relaxation times were measured by inversion recovery method in varying inversion time (TI). T2 relaxation times were measured using CPMG (Carr-Purcell-Meiboon_Grill) pulse sequence for multiple spin-echo measurements. The transmetallation stability study was performed at 3.0 T MR unit (128MHz, Siemens) by measurement of the evolution of water proton longitudinal relaxation rate (R1p). The binding study was carried out by mixing GdL and Gadovist® with beta-amyloid oligomer per each and the mixture was incubated for 24 h at room temperature. The left solution was removed and washed out with DMEM two times. After centrifugation, the pellet was dissolved in DMEM solution with 10% DMSO and T1- weighted image was acquired at 9.4 T. The imaging parameters for T1- weighted image are as follows; TE 6.7 ms, TR 100 ms, FOV 3ⅹ3 cm, matrix size 128ⅹ128, slice thickness 1 mm, Average 2, scan time 25 s 600 ms. In vivo MR image on 5XFAD model was acquired after gadolinium complex administration by intracerebroventricular (icv) injection with following parameters; TR 670 ms, TE 6.7 ms, FOV 2ⅹ2 cm, matrix size 128ⅹ128, slice thickness 0.5 mm, Average 4, scan time 5 min 43s.Results and Discussion
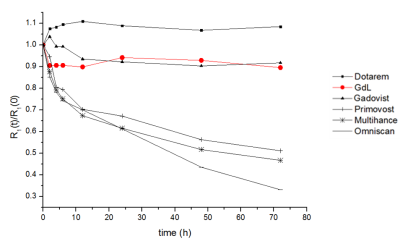
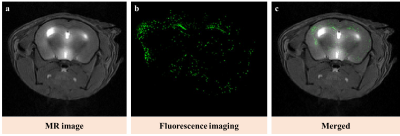
Figure 1 shows the structure of GdL and its relaxivity values at 1.5 and 9.4 T. GdL has high relaxivities even at high field (9.4 T) such as r1 = 4.60 and r2 = 5.71 mM-1s-1. Kinetic inertness study in figure 2 proved that GdL can tolerate dissociation at competitive environment with zinc chloride. T1-weighted images of Aβ oligomer showed signal difference between Gadovist® (signal intensity: 18.71 A.U) and GdL (signal intensity: 28.10 A.U) (Figure 3). This implied that GdL has a specific binding affinity with beta-amyloid oligomer. In vivo MRI also confirmed the specificity of GdL with beta-amyloid oligomer presenting the significant signal enhancement at the region of beta-amyloid deposition of the brain (Figure 4a). Fluorescence imaging of stained beta-amyloid plaque with thioflavin S was matched well with the enhanced region in a MR image and it also supported that GdL shows a specificity on beta-amyloid (Figure 4b,c).Conclusion
We successfully prepared a new MRI contrast agent for beta-amyloid imaging. GdL shows high relaxivities even at high field and comparable kinetic stability. It also shows signal enhancement either in vitro beta-amyloid oligomer condition and in vivo MR imaging on 5XFAD alzheimer’s disease model. This proved that GdL was used as a successful imaging agent for beta-amyloid.Acknowledgements
No acknowledgement found.References
[1] Chai Lean Teoh et al, J. Am. Chem. soc., 2015, 137(42), 13503-13509
[2] Masahiro.Ono et al, Bioorg. Med. Chem., 2007, 15, 6802-6809
[3] Kwok Kin Cheng et al, Biomaterials, 2015, 44, 155-172
Figures