3987
A custom-designed 1H saddle coil for CEST imaging at 14.1 Tesla1Laboratory for functional and metabolic imaging (LIFMET), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 2School of Health Sciences, University of Applied Sciences and Arts of Western Switzerland (HES-SO), Geneva, Switzerland, 3Department of Psychiatry, McGill University, Montreal, QC, Canada, 4Department of Radiology, Université de Lausanne (UNIL), Lausanne, Switzerland, 5Department of Radiology, Geneva University Hospital (UNIGE), Geneva, Switzerland
Synopsis
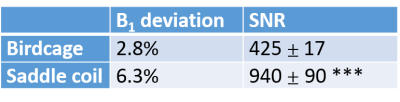
We propose an alternative RF method for small animal CEST imaging at ultra-high magnetic field by using a 1H saddle coil instead of a quadrature birdcage coil. The coils create similar homogeneous B1 maps with an 2.2x increase of SNR for the saddle coil. Both coils show a similar degree of precision when tested for in vitro glycoCEST imaging. In rodent skeletal muscle the saddle coil offers a good performance with respect to the sensitivity for glycoCEST imaging.
Purpose
Chemical Exchange Saturation Transfer (CEST) uses the chemical exchange of protons of water with protons of chemical entities to produce molecular imaging contrast. High magnetic field is generally advantageous for CEST imaging in terms of spectral dispersion, bulk water T1 relaxation and Signal to Noise Ratio (SNR). Spatial homogeneity of the radio-frequency (B1) field over the ROI is indispensable for CEST effect interpretation, hence volume coils and especially birdcage coils are preferred. However, due to the large dimensions of this coil design, they do not provide an ideal SNR in the volume of interest. In this study, we therefore propose a saddle coil for specific 14.1T CEST applications to increase SNR with an optimized ROI-adjusted design, but also to facilitate animal manipulation and preserve B1 field homogeneity. We evaluated the coil performance for in vitro and in vivo muscular glycoCEST imaging. Muscle glycogen CEST is a particularly challenging measurement in terms of SNR due to the proximity of the glycogen hydroxyl group resonance frequency to the water frequency, the related limited fulfillment of the slow proton exchange regime condition (Δω>>κex) and the smaller concentrations of glycogen found in skeletal muscle as compared to liver [1,2].Materials and Methods
All experiments were conducted
with a 14.1T MR Varian system with a 26cm horizontal bore (Agilent, Oxford, UK).
Results were acquired with a custom-build 1H high-pass quadrature
birdcage coil with 16 legs [3,4] (diameter = 50mm, length = 27mm) and a custom-designed
single channel 1H saddle coil with 4 rods (diameter = 34mm, leg
length = 22mm, angular aperture = 120° [5]). Due to the short wavelength at 14.1T
the saddle coil was segmented in 4 resonant sections by 2.2pF capacitors. B1
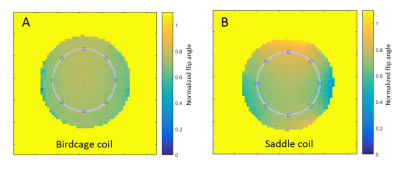
maps were created from gradient echo images (α=60°/120°) according to the double
angle method [6,7].
In vivo and in vitro CEST images
were acquired with a train of Gaussian saturation pulses coupled to a segmented
gradient echo sequence [1]. Comparisons were established with a 50mM
glycogen phantom (glycogen from oyster, Sigma-Aldrich, Switzerland)
of 14mm diameter. It is comparable in size and loading to a rat leg muscle. Spatial
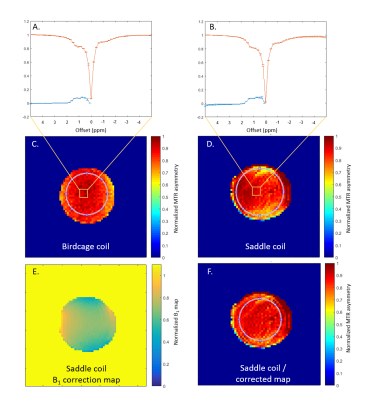
B1 inhomogeneity was accounted for by correcting the Magnetization Transfer
Ratio Asymmetry (MTRasym) maps with normalized B1 maps. The MTRasym values were
normalized to account for possible discrepancies in actual flip angles. The
back upper leg of a rat was scanned at 14.1T inside the saddle coil.Results
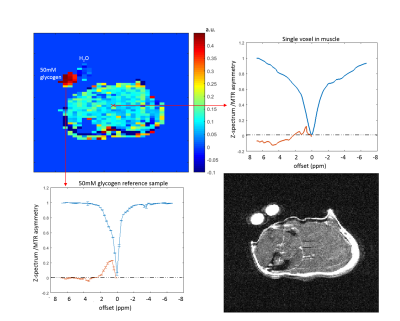
The saddle coil increased the SNR with respect to the birdcage coil by a factor of 2.2 in a 50mM glycogen phantom (Fig.1, table1). Furthermore, the analysis of MTRasym maps of a homogeneous glycogen phantom showed a coefficient of variation of 5.3% for the birdcage coil and 8.3% for the saddle coil. To compensate for higher B1 inhomogeneities created by the saddle coil (table1) a correction was applied to the MTRasym map resulting in a coefficient of variation of 5.2%, thus comparable with the raw birdcage coil measurements (Fig.2). The power needed to achieve a 90° flip angle could be reduced by 5dB for the saddle coil. In vivo muscular glycoCEST measurements showed no CEST effect in the H2O reference and a well-defined CEST effect in the 50mM glycogen reference (Fig.3). Glycogen and creatine were clearly visible in the MTRasym curve of a single voxel at 1.8ppm and 1.2ppm, respectively. GylcoCEST imaging derived from the sum of MTRasym values from [1-0.5]ppm is nearly homogeneous in muscle tissues.Discussion
The saddle coil increases SNR by more than double depending on the dielectric properties of the loading material. The decrease in power needed by the saddle coil allows one to create narrower pulses and is advantageous for future 1H MRS measurements. Finally, glycoCEST imaging of a rodent leg muscle was achieved using the saddle coil (Fig.3). The results compare well with measurements published previously using a birdcage coil (1). The performance of the saddle coil on the muscle provided optimized sensitivity for MTRasym quantification and offered high quality data with a resolution of 0.877μl.Conclusion
We conclude that a saddle coil can be an advantageous alternative to the often-used birdcage coil for preclinical ultra-high field CEST applications, which benefits from increased SNR without suffering from loss of B1 homogeneity. Associated with the inherent higher NMR polarization at 14.1T this increased sensitivity opens the way to faster and multi-slice CEST imaging, of particular interest for the challenging measurement of glycoCEST. Moreover, the simplified open design of the saddle coil makes it a robust alternative, facilitating animal positioning.Acknowledgements
We thank Andrew Webb for most insightful discussions; as well as Da Silva Analina Raquel, Mario Lepore and Mitrea Stefanita-Octavian for their support with the in vivo experiments. This study was supported by the Leenaards and Jeantet foundation.References
1. E. Vinckenbosch, H. Lei, N. Kunz, M. Dehghani and R. Gruetter. Muscular glycogen detection with CEST imaging in living rat at 14.1T. 25th Annual Meeting of ISMRM, Honolulu, 2017.
2. P.C.M. Van Zijl, C.K. Jones, J. Ren, C.R. Malloy, A.D. Sherry, MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST). Proceedings of the National Academy of Sciences, 104(11), 2007.
3. M. Dehghani M., A. Magill W., Y. Pilloud, N. Kunz and R. Gruetter. Transmit volume coil-receive surface coil for proton operating at 14 Tesla. International Society for Magnetic Resonance in Medicine, Toronto, Canada (2015).
4. T. Cheng, A.W. Magill, A. Comment, R. Gruetter and H. Lei. Ultra-high field birdcage coils: a comparison study at 14.1 T. Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE, 2014.
5. D.M. Ginsberg and M.J. Melchner. Optimum Geometry of Saddle Shaped Coils for Generating a Uniform Magnetic Field. Review of Scientific Instruments 41, 122, 1970.
6. R. Stollberger, P. Wach, G. McKinnon, E. Justich, F. Ebner. Rf‐field mapping in vivo. Proceedings of the 7th Annual Meeting of SMRM, San Francisco, CA, USA, 1988.
7. E.K. Insko and L. Bolinger. B1 mapping. Proceedings of the 11th Annual Meeting of SMRM, Berlin, Germany, 1992.
Figures