3822
Comparison of Cartesian MR thermometry approaches for focused ultrasound brain applications1Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 2Siemens Healthineers, Baltimore, MD, United States, 3Siemens Healthineers, Boston, MA, United States, 4Siemens Healthineers, Salt Lake City, UT, United States
Synopsis
Magnetic resonance guided focused ultrasound using MR thermometry (MRT) for therapy guidance is a promising thermal therapy for a wide range of neurological disorders. The current MRT method acquires a single 2D slice with low readout bandwidth to increase SNR and MRT precision, compensating for the fact that the body coil is used to receive during treatments. In this work we investigate trade-offs between 2D and 3D GRE and EPI approaches of MRT for brain FUS applications. A comparison between using the body coil and two 4-channel flex coils is also performed.
Introduction
Transcranial magnetic resonance guided focused ultrasound (tcMRgFUS) is a totally non-invasive treatment modality which is currently used to treat essential tremor and is being investigated for a wide variety of neural disorders such as Parkinson’s disease, psychiatric disorders and neuropathic pain (1–3). MR thermometry (MRT), using the proton resonance frequency shift method, is used to monitor the treatments as it provides non-invasive temperature measurements with high spatio-temporal resolution and accuracy. The current MRT approach used for the Exablate Neuro system (Insightec, Tirat Carmel, Israel) is to dynamically acquire a single 2D slice. Due to the complexity of adding dedicated RF receive coils to the FUS system, the body coil is used for signal receive. To improve SNR (and hence MRT precision since $$$SNR\propto1/\sigma_T\propto\sqrt{sampling time}$$$) a low readout bandwidth (~40-50 Hz/pixel) is utilized. This results in a long sampling time and a high sampling duty cycle. However, this also results in significant off-resonance shifts in the readout direction due to, e.g., fat/water and temperature change. Further, the single 2D slice is not able to monitor the full focal spot region.
Various volumetric 2D and 3D methods utilizing higher sampling bandwidth have been proposed to reduce off-resonance shift and increase coverage (4–7). Many of these approaches utilize advanced pulse sequences with non-Cartesian sampling and/or dedicated reconstruction methods, which are often not available on MRI scanners from all vendors and are not well suited for real-time reconstruction. In this work we compare 2D and 3D Cartesian approaches using widely available pulse sequences and straightforward reconstruction for improved MRT during transcranial MRgFUS.
Methods
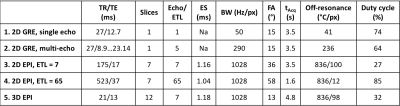
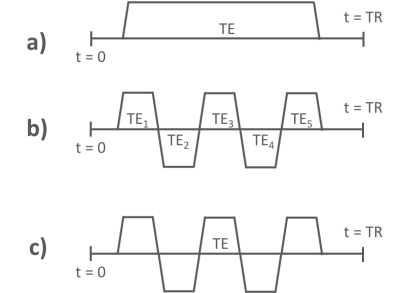
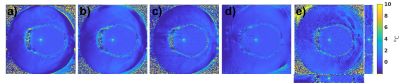
5 different MRT approaches were compared using widely available 2D GRE and 2D and 3D echo planar imaging (EPI) pulse sequences, Table 1. 1) Using the current clinical protocol of a single 2D slice with low bandwidth (Figure 1a). 2) Keeping everything the same as in protocol 1) but splitting the long single readout into 5 bi-polar echoes (8), each with an increased readout bandwidth to minimize off-resonance effects (Figure 1b). 3) Turning the multi-echo approach in 2) into a segmented EPI sequence (Figure 1c) to traverse k-space faster. The improved speed was traded for coverage such that seven 2D slices were acquired in the same time with an echo train length (ETL) of 7. 4) Increasing the ETL in 3) to sample half of all phase encoding lines after each excitation, and hence decreasing acquisition time while sampling the same seven slices and utilizing a longer TE, resulting in improved MRT precision. 5) 3D EPI approach sampling 12 slices with ETL = 7.
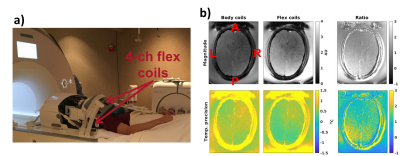
All experiments were performed on a 3T scanner (Magnetom PrismaFIT, Siemens Healthcare, Erlangen, Germany) scanning a healthy volunteer (without FUS heating) and a gelatin-filled ex-vivo skull (with FUS heating, 350 W for 40 s) in the Exablate Neuro system. A comparison between using the body coil and two flex coils (placed anteriorly and posteriorly) was performed, Figure 2. All protocols were scanned for 1 minute and the MRT precision was calculated as the voxel-wise standard deviation through time, and then averaged over a region-of-interest. All PRFS maps were calculated using alpha = -0.01 ppm/°C and single baseline subtraction followed by referenceless reconstruction by fitting a second order polynomial (9).
Results
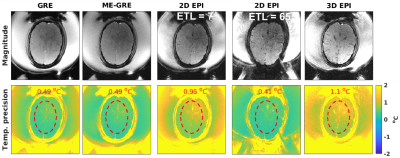
Figure 2b shows that the MRT precision increased by up to a factor of 2x by using the flex coils. In Figure 3 the in-vivo magnitude and MRT precision maps for all protocols are shown. Figure 4 shows comparisons of sonications in the ex-vivo skull, with an example of the increased coverage achieved by 3D imaging.Discussion and Conclusions
The 2D EPI protocol with long ETL showed the best precision due to the long TE and sampling time, but also demonstrated geometric distortions. The single- and multi-echo approaches had the same precision, showing that optimally combining multiple echoes can compensate for the lower sampling duty cycle achieved due to switching of the readout polarity in the multi-echo approach. The 2D and 3D EPI approaches with ETL=7 showed lower MRT precision but provided more complete coverage and had at least 2.4x smaller off-resonance shifts than the currently used single-echo approach. Utilizing 3D MRT it is possible to fast and accurately localize the focal spot in all three directions with a single sonication. 3D MRT will further be able to detect focal spot shifts during the treatment, so that the maximum temperature is always detected and measured.Acknowledgements
This work was supported by Siemens Healthcare, the Mark H. Huntsman endowed chair, and NIH grant R01EB013433, and S10OD018482.References
1. Ghanouni P, Pauly KB, Elias WJ, Henderson J, Sheehan J, Monteith S, Wintermark M. Transcranial MRI-Guided Focused Ultrasound: A Review of the Technologic and Neurologic Applications. Am. J. Roentgenol. [Internet] 2015;205:150–159. doi: 10.2214/AJR.14.13632. 2. Khanna N, Gandhi D, Steven A, Frenkel V, Melhem ER. Intracranial applications of mr imaging-guided focused ultrasound. Am. J. Neuroradiol. 2017;38:426–431. doi: 10.3174/ajnr.A4902. 3. Meng Y, Suppiah S, Mithani K, Solomon B, Schwartz ML, Lipsman N. Current and emerging brain applications of MR-guided focused ultrasound. J. Ther. Ultrasound 2017;5:1–9. doi: 10.1186/s40349-017-0105-z. 4. Todd N, Adluru G, Payne A, DiBella EVR, Parker D. Temporally constrained reconstruction applied to MRI temperature data. Magn. Reson. Med. 2009;62:406–19. 5. Jonathan S V., Grissom WA. Volumetric MRI thermometry using a three-dimensional stack-of-stars echo-planar imaging pulse sequence. Magn. Reson. Med. 2018;79:2003–2013. doi: 10.1002/mrm.26862. 6. Marx M, Ghanouni P, Butts Pauly K. Specialized volumetric thermometry for improved guidance of MRgFUS in brain. Magn. Reson. Med. [Internet] 2017;78:508–517. doi: 10.1002/mrm.26385. 7. Fielden SW, Feng X, Zhao L, Miller GW, Geeslin M, Dallapiaza RF, Elias WJ, Wintermark M, Butts Pauly K, Meyer CH. A spiral-based volumetric acquisition for MR temperature imaging. Magn. Reson. Med. [Internet] 2017;Early view:1–6. doi: 10.1002/mrm.26981. 8. Butts-Pauly K, Marx M, Ghanouni P. Multi-echo MR thermometry compared to single-echo MR thermometry in the treatment of essential tremor. In: International Symposium on Focused Ultrasound. ; 2016. p. BR-17. 9. Rieke V, Vigen KK, Sommer G, Daniel BL, Pauly JM, Butts K. Referenceless PRF shift thermometry. Magn. Reson. Med. 2004;51:1223–1231. doi: 10.1002/mrm.20090.Figures