3696
Reduced brain functional connectivity induced by generalized pain identified by mice Rs-fMRI1Department of Psychiatry, Mcgill University, Montreal, QC, Canada, 2Department of Pharmacology and Therapeutic Innovation, Nagasaki University Institute of Biomedical Sciences Nagasaki, Nagasaki, Japan, 3Department Neurology & Neurosurgery, Mcgill University, Montreal, QC, Canada
Synopsis
Fibromyalgia (FM) is a disorder characterized by generalized pain. The acid saline-induced muscle (ASM) model is considered an acceptable animal model of FM with widespread chronic pain. Here, we investigated the impact of FM-like pain on neural communication, using the ASM model with non-invasive mouse resting state fMRI. We found that generalized pain reduces functional connectivity at the level of periaqueductal gray and retrosplenial cortex, two regions related to pain processing and FM. Moreover, we found a positive correlation between pain sensitivity measured by Von Frey test and the intensity of FC reduction at individual subject level.
Target audience
Pain, fibromyalgia, neuroimaging, translational studies
Purpose
Fibromyalgia (FM) is a disorder involving central and generalized pain, which affects approximately 2% population ratio in developed countries. FM patients are weakly responsive to commonly used analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids. Although there is an increasing body of evidence supporting a role for peripheral small fiber neuropathy as well as central mechanisms as potential causative factors in FM, etiological information of FM remains limited delaying diagnosis and therapy1. Previously, others and we developed the acid saline-induced muscle (ASM) mouse pain model2, characterized by chronic and widespread pain, and considered a FM-like chronic muscle pain model. Here, we use this animal model to study neuronal communication at whole brain level using non-invasive resting-state functional magnetic resonance neuroimaging (Rs-fMRI). We test whether mice receiving intramuscular PH7.2 (control) and PH4.0 (FM) treatment show distinct functional connectivity (FC) patterns. We found a reduction of FC in pain-related anatomical regions. Moreover, this reduction was positively correlated with pain levels at individual subject level.
Methods
Animal preparation: Acid saline (PH7.2 or PH4.0, adjusted by 0.1 N HCL) was injected in the left gastrocnemius muscle of each mouse twice, with a 5 days interval, to induce generalized pain as previously described2 (n=10 C57/BL6 male mice/treatment group). Five days after the second injection, MRI was performed under continuous Isoflurane anesthesia (5% outside and 2% inside the scanner). Image acquisition: fMRI images were acquired in a 7 tesla MRI scanner with a cryoprobe using EPI sequence (TE=1ms; TR=1.5s) matrix 128×80, 15 axial slices, field of view 1.39×1.25 cm2 and 400 volumes for a total acquisition time of 10 minutes. Image preprocessing: time series were denoised, motion corrected, co-registered, spatially normalized with Allen brain template, smoothed (FWHM of 0.3×0.3×1 mm3) and bandpass filtered (0.01-0.1Hz)3. ICA analysis: Independent Component Analysis (ICA) was performed as in Ref3. Seed-to-seed analysis: was performed for 14 pain-related brain regions. Prefrontal Cortex (PFC), Anterior Cingulate Area (ACA), Caudate Putamen (CP), Insular (Ins), Amygdala (Amy), Habenular (Hab), Hypo thalamus (Hyp), Somatosensory cortex (SS), Thalamus (TH), Retrosplenial (RSP), Hippocampus (HIP), Periaqueductal gray (PAG), Superior colliculus (SC) and Parabrachial nucleus (PBN) seeds were generated manually based on the mouse Allen brain atlas, and mean time series were extracted for seed-to-seed FC analysis including statistical significance test (t-test). Seed-voxelwise analysis: The PAG seed was used for this analysis. Mean time series were extracted and used to compute FC with all the voxels. Group difference significance was set at a threshold Z score 5 (significance/alpha level 0.001, one tail). All results were family-wise error rate (EWER) corrected following cluster with significance/alpha level 0.05. Pain Sensitivity test: Von Frey testing was performed on all the animals to measure pain sensitivity to both sides of paw, two days and one month after scanning4. Mean results of both paws were used to quantify the hyperalgesia induced by the ASM. Results are expressed pressure (g) for paw withdrawal. Correlation between imaging and behavioural data: Fisher's transformed Z scores of correlation coefficients for PAG-RSP FC, and paw withdrawal thresholds (left & right mean, g) were correlated using Pearson analysis.
Results
Using ICA, we found that generalized pain induces a robust reduction of FC (PH7.2>PH4.0), with strongest changes at the level of the RSP, as well as significant modification in the SC and midbrain (Figure 1). Next, we tested FC modifications across 14 pain-related regions using seed-to-seed analysis and found that PAG and RSP seeds showed the highest number of reduced correlated connectivity with other seeds (PAG, 7; RSP, 6) (Figure 2). Then, we performed PAG seed-to-voxel FC analysis and found that PAG seed-based connectivity was highly reduced with several brain regions including ACA (center of salience network), RSP, SC, Hab and Th (Figure 3). Finally, we found a positive correlation between RSP-PAG FC reduction and FM-like hyperalgesia at individual subject level, which was close to significance (Figure 4).
Discussion and conclusion
Two previous human studies suggest that a reduction of PAG connectivity is related to clinical manifestations of FM5 and that RSP is involved encoding Self-Referential Pain Catastrophizing of FM6. Altogether, our results are in full concordance with the human literature, as we found that PAG and RSP are predominantly modified in the ASM mouse model. Our findings have two implications. First our Rs-fMRI data represent a strong validation for ASM to be a valid FM model in the mouse. Second, pharmacological or genetic manipulations may now be further developed using ASM together with MRI towards translational studies for human suffering from FM.Acknowledgements
US National Institutes of Health (National Institute of Drug Addiction, grant # 05010 and National Institute on Alcohol Abuse and Alcoholism, grant #16658), the Canada Fund for Innovation and the Canada Research Chairs for financial support. McGill University HBHL grant to H.U.
References
[1] H. Ueda and H. Neyama, Neurobiology of Pain 1 (2017) 16–25
[2] K.A. Sluka et al, Muscle Nerve, 24 (1) (2001), pp. 37-46
[3] Mechling et al, PNAS (2016), 1-6
[4] JR Deuis et al, Frontiers in Molecular Neuroscience. 2017;10:284
[5] M-A Coulombe et al, Frontiers in Neuroanatomy. 2017;11:47
[6] Lee J et al, Arthritis Rheumatol. 2018 Mar 26
Figures
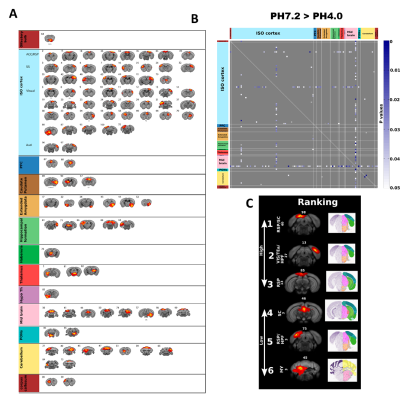
FC reduction using unbiased independent component analysis (ICA); (A) ICA identifies 97 components, displayed here in anatomical rostro-caudal order, (B) Group comparison (PH7.2 > PH4.0) significant p values by t-test (P<0.05, n=10, one tail) following the same anatomical order of A, (C) Top 6 components with highest number of significant FC reduction are shown. Corresponding 2D slice from Allen brain atlas are also presented showing the anatomical region. [Component identification number is shown on top. Nucleus Accumbens (NAc), Periaqueductal gray (PAG), Ventral tegmental area (VTA), Habenular(Hab), Anterior cingulate area (ACA), Retrosplenial (RSP), Thalamus (Th), Hippocampus (Hipp) and Superior colliculus (SC)]
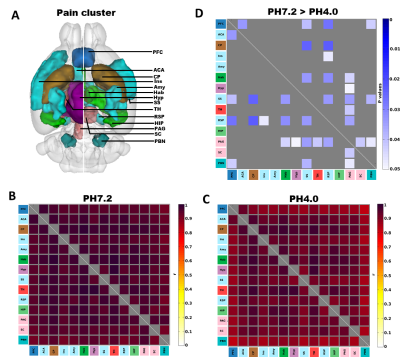
Seed-to-seed analysis shows highest modifications in RSP and PAG; (A) 3D illustration of the 14 selected pain-related seeds. Mean correlation coefficient for (B) PH7.2 and (C) PH4.0 groups, (D) Group comparison (PH7.2 > PH4.0) p values using one tail t-test (P<0.05, n=10) shows seeds with higher functional connectivity in PH7.2 compared to PH4.0 groups. The color bar indicates the corresponding significant P-value scaling.[ Prefrontal cortex (PFC), Anterior Cingulate Area (ACA), Caudate Putamen (CP), Insular (Ins), Amygdala (Amy), Habenular (Hab), Hypo thalamus (Hyp), Somatosensory cortex (SS), Thalamus (TH), Retrosplenial (RSP), Hippocampus (HIP) Periaqueductal gray (PAG), Superior colliculus (SC) and Parabrachial nucleus (PBN)]
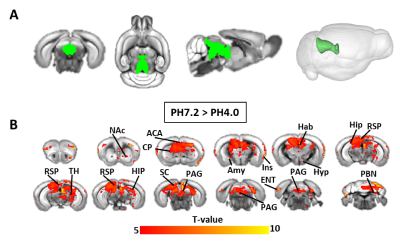
PAG seed to whole brain connectivity analysis; (A) both 2D and 3D representation of PAG seed (green); (B)PAG seed based FC Group comparison (PH7.2 > PH4.0) t values by t-test (P<0.001, n=10 group, FWER corrected) shows voxels with higher functional connectivity in PH7.2 compared to PH4.0 groups. [ Nucleus Accumbens (NAc), Anterior Cingulate Area (ACA), Caudate Putamen (CP), Insular (Ins), Amygdala (Amy), Habenular (Hab), Hypo thalamus (Hyp), Thalamus (TH), Retrosplenial (RSP), Hippocampus (HIP) Periaqueductal gray (PAG), Superior colliculus (SC) , Entorhinal area (ENT) and Parabrachial nucleus (PBN) ]
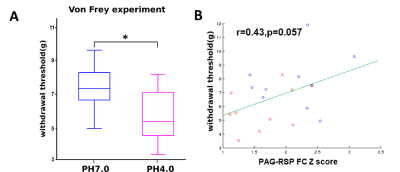
Positive correlation between PAG-RSP FC and pain sensitivity; (A) Pain sensitivity was measured using the Von Frey Test. Data are expressed as paw withdrawal threshold (average of both sides) in gram and are represented using a box plot with t-test (N=10/10, *P<0.05); (B) Positive correlation between hyperalgesia and PAG-RSP FC (Z scores) (PH7.2 (blue) and PH4.0 (red), N=10/10) is shown as a scatter plot and regression line.