3484
Machine learning can boost the acquisition and estimation of anomalous diffusion model1Center for MRI Research, Peking University, Beijing, China, 2MR Research China, GE Healthcare, Beijing, China
Synopsis
The departure from mono-exponential decay of the diffusion-induced signal loss has promoted the research of anomalous diffusion in MRI. It has been found that anomalous diffusion models offer substantial advantages over the conventional method in clinical applications. However, these models require more diffusion weightings for complicated estimation procedure, which prevents its further application. In this study, we demonstrated that machine learning can be applied to accelerate the estimation of anomalous diffusion parameters. Furthermore, feature selection was used to identify the most relevant signals, thus helping to reduce the extensive sets of diffusion weightings.
Introduction
In recent years, anomalous diffusion models have been proposed to address the phenomenon that diffusion-induced MRI signals deviate from the mono-exponential form1. Clinical feasibility studies have been conducted and those anomalous diffusion models have been found to outperform the conventional diffusion MRI (dMRI) method2–6. However, numerous diffusion weightings and complicated estimation procedures are required to obtain those models. It leads to long acquisition and estimation time. Recently, machine learning techniques have been applied in dMRI researches7,8. Inspired by these studies, here we propose a machine learning based framework to efficiently estimate anomalous diffusion parameters. Moreover, the most important diffusion weightings was identified with the feature selection technique9, which is useful to prune the extensive sets of diffusion weightings.Methods
The fractional motion (FM) model, which is considered to be appropriate to describe the anomalous diffusion in biological tissues10, was selected for demonstration. The Noah exponent (α) and the Hurst exponent (H) characterize the FM model11,12. The FM-based dMRI signal with Stejskal-Tanner (S-T) pulse gradients can be written as
$$S/S_0=\exp(-\eta \cdot D_{\alpha,H}\cdot\gamma^{\alpha}G^{\alpha}\Delta^{\alpha+\alpha H})$$
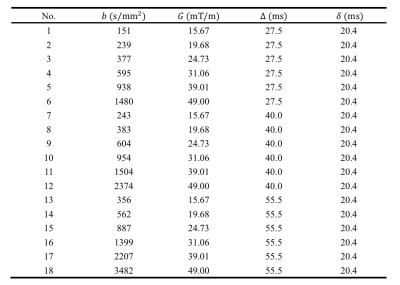
where $$$D_{\alpha,H}$$$ is the generalized diffusion coefficient, γ is the gyromagnetic radio, G is the diffusion gradient amplitude, and Δ is the diffusion gradient separation time. η is a dimensionless number that can be calculated with α, H, Δ, and the diffusion gradient duration (δ). The acquisition protocol in previous studies was evaluated here5,6. Details of the 18 non-zero diffusion weightings can be found in Table 1.
Random forest (RF) regression was used to learn the mapping between the anomalous diffusion parameters and the dMRI signals13. The RF regressors were implemented in the scikit-learn toolkit and each regressor contained 200 trees with maximum depth determined during training14. To train and validate the regressors, diffusion signals from 60000 voxels were simulated, with FM-related parameters randomly selected in the ranges: $$$\alpha\in(1,2]$$$, $$$H\in(0,1)$$$. $$$D_{\alpha,H}$$$ was drawn from a log-norm distribution ($$$\mu=-5.70, \sigma=1.15$$$) to mimic the results in previous studies5,6. Rician noise was then added and five sets of signals were generated: noise-free, SNR=50, SNR=40, SNR=30, and SNR=20 (for the b=0 signals). The RF regressors were trained on 48000 voxels and the remaining 12000 were used for testing. Model fitting was also performed on the test dataset for comparison.
Results
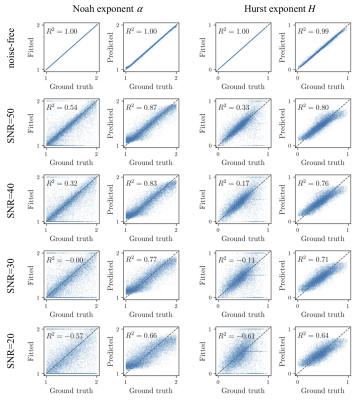
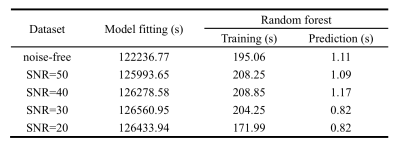
Figure 1 shows the scatter plots of the Noah exponent α and Hurst exponent H against the values calculated by fitting and predicted by RF. Coefficient of determination (R2) was used here to measure how well the estimated values approximate the ground truth. Although there is a one-to-one correspondence between the fitted and ground truth values when data is noise-free, The RF outperformed the model fitting method when there is noise. It should be noted that the fitting method sometimes produced the boundary values rather than the close values to ground truth, while the RF performed well in all situations. The computation time is summarized in Table 2. The RF method completed the estimation much faster than traditional fitting.
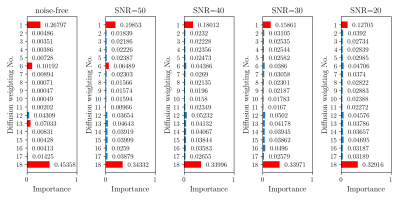
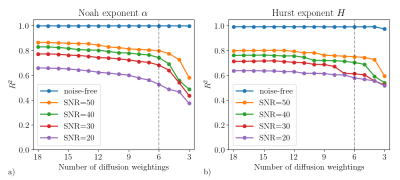
Figure 2 illustrates the feature importance in the RF regressors for the 18 diffusion weightings. Only a minority of weightings are decisive. Therefore, irrelevant weightings can be abandoned, which is very helpful to reduce the acquisition time. As Figure 3 indicated, the RF regressors which were constructed on the most important 6 signals performed similarly to those based on all signals.
Discussion and Conclusion
In this study, we applied random forest regression to accelerate the estimation of anomalous diffusion parameters. Compared with conventional model fitting method, the RF regressors can estimate the anomalous diffusion parameters faster and more accurately. Although, there are other machine learning methods (e.g. deep learning) can be applied to this issue, the random forest is proven to identify the most relevant signals, which is useful to reduce the extensive sets of diffusion weightings.Acknowledgements
No acknowledgement found.References
1. De Santis S, Gabrielli A, Palombo M, et al. Non-Gaussian diffusion imaging: a brief practical review. Magn Reson Imaging 2011;29:1410–6.
2. Kwee TC, Galbán CJ, Tsien C, et al. Intravoxel water diffusion heterogeneity imaging of human high-grade gliomas. NMR Biomed 2010;23:179–87.
3. Sui Y, Xiong Y, Jiang J, et al. Differentiation of low-and high-grade gliomas using high b-value diffusion imaging with a non-Gaussian diffusion model. Am J Neuroradiol 2016;37:1643–1649.
4. Karaman MM, Sui Y, Wang H, et al. Differentiating low- and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values. Magn Reson Med 2016;76:1149–57.
5. Xu B, Su L, Wang Z, et al. Anomalous diffusion in cerebral glioma assessed using a fractional motion model. Magn Reson Med 2017;78:1944–9.
6. Xu B, Su L, Wang Z, et al. Anisotropy of anomalous diffusion improves the accuracy of differentiating low- and high-grade cerebral gliomas. Magn Reson Imaging 2018;51:14–9.
7. Golkov V, Dosovitskiy A, Sperl JI, et al. q-Space Deep Learning: Twelve-Fold Shorter and Model-Free Diffusion MRI Scans. IEEE Trans Med Imaging 2016;35:1344–51.
8. Nedjati-Gilani GL, Schneider T, Hall MG, et al. Machine learning based compartment models with permeability for white matter microstructure imaging. NeuroImage 2017;150:119–35.
9. Guyon I, Elisseeff A. An introduction to variable and feature selection. J Mach Learn Res 2003;3:1157–1182.
10. Fan Y, Gao J-H. Fractional motion model for characterization of anomalous diffusion from NMR signals. Phys Rev E 2015;92:012707.
11. Eliazar II, Shlesinger MF. Fractional motions. Phys Rep 2013;527:101–29.
12. Burnecki K, Weron A. Fractional L\’evy stable motion can model subdiffusive dynamics. Phys Rev E 2010;82:021130.
13. Breiman L. Random forests. Mach Learn 2001;45:5–32.14. Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: Machine Learning in Python. J Mach Learn Res 2011;12:2825–30.
Figures