3432
3D Diffusion MRI of Perivascular Fluid Movement: Towards Non-Invasive Mapping of Glymphatic Function.1Centre for Advanced Biomedical Imaging, Division of Medicine, University College London, London, United Kingdom, 2The Francis Crick Institute, London, United Kingdom, 3Neuroradiological Academic Unit, Department of Brain Repair and Rehabilitation, UCL Queen Square Institute of Neurology, London, United Kingdom, 4Leonard Wolfson Experimental Neurology Centre, UCL Queen Square Institute of Neurology, London, United Kingdom
Synopsis
Within the glymphatic system, CSF is transported in a network of perivascular channels where it exchanges with ISF to drive drainage of unwanted solutes, like amyloid- β, out of the brain. Perivascular channel impairment may be an early biomarker of neurodegenerative processes. Here, we present a pilot study for 3D non-invasive assessment of glymphatic function in the rat brain using ultra-long echo time diffusion MRI. We show that this technique is sensitive to the fluid movement in downstream perivascular channels that drives glymphatic inflow.
Introduction
Efficient waste removal from the brain is essential to maintain normal physiology and function$$$^{1}$$$. The glymphatic pathway describes a network of perivascular channels that facilitate rapid cerebrospinal fluid (CSF) transport and exchange with the brain’s interstitial fluid (ISF), for parenchymal waste clearance$$$^{2}$$$. The continuous interchange between CSF and ISF washes unwanted solutes towards larger central veins and lymphatic vessels to eventually be removed from the CNS$$$^{3}$$$. Thus, perivascular channels play a crucial role in CSF-mediated clearance of potentially toxic molecules, such as amyloid-β$$$^{4}$$$. Perivascular channel impairment may lead to a build-up of toxic molecules in the aging brain and eventually, the onset of neurodegenerative diseases such as Alzheimer’s disease (AD). As such, the perivascular space (PVS) represent a promising target for non-invasive imaging biomarkers of glymphatic function. Recently, we have introduced the first non-invasive technique to assess the glymphatic pathway using ultra-long echo time (TE) diffusion-weighted MRI sequences in the rat brain$$$^{5}$$$. This technique captured the dynamics of CSF movement within the PVS of an upstream glymphatic target; the middle cerebral artery (MCA) at the base of the rat brain. Moreover, the application of this technique was limited to single slice 2D acquisitions which restricted the measurement to a small segment of the glymphatic pathway. Here, we use a 3D acquisition working towards whole brain mapping of PVS function across the glymphatic network of the rat brain. As such, in this pilot study, we examine more downstream segments of PVS anatomy, closer to the eventual site of CSF delivery to the tissue. In addition, we investigate whether cardiac cycle-related vessel pulsatility changes the recorded PVS fluid movement during arterial pulsation and diastole.Methods
A healthy male Sprague Dawley rat was anaesthetised and scanned on an Agilent 9.4T system (free-breathing, 2% isoflurane in 0.2L/min medical air and 0.8L/min O2).
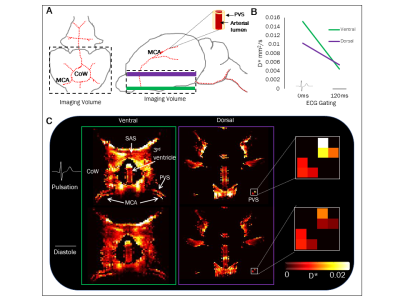
Diffusion-Weighted Imaging: An imaging volume was positioned at the base of the brain (see Figure 1A). A fast-spin-echo 3D diffusion weighted sequence was acquired with the following sequence parameters: TR = 4s, effective TE = 133ms, echo train length = 32, echo spacing = 11ms, FOV = 30mm x 30mm x 8mm, matrix (RO x PE x PE2) = 192 x192 x 16, 4 averages, b0 + one direction (ventrodorsal), b-value = 43 s/$$$mm^{2}$$$.
ECG-Gating: A three lead electrode was used to measure ECG signals in the bore of the magnet. Image acquisition was gated to the ECG signal to capture fluid movement in PVS during vessel pulsation and diastole. As such, image acquisition began either directly after the R-wave (i.e. during pulsation) or with a 120ms delay (i.e. during diastole).
Analysis: Region of interests were manually drawn around the PVS surrounding the left and right branches of the MCA at the ventral and dorsal aspects of the blood vessel (see Figure 1A).
The pseudo-diffusion coefficient (D*) was then calculated using the following equation:
$$S = S0 exp(-bD*)$$
where S is the measured signal at b=43s/$$$mm^{2}$$$ and S0 is the signal taken from the b=0 image.
Results
Figure 1A shows a schematic of the rat brain in both axial and sagittal orientations. These show the origin of the MCA from the base of the brain near the circle of Willis (CoW) and where it branches symmetrically around the rostral-lateral outer edges of the brain. Figure 1C shows the pseudo-coefficient (D*) maps from the upstream (green) and more downstream (purple) targets of the PVS surrounding the MCA during arterial pulsation (0ms) and diastole (120ms). The D* maps and graph in Figure 1B show, in this animal, that perivascular fluid in both regions of the MCA has a greater D* during arterial pulsation compared to diastole. Moreover, the recorded D* values of the downstream PVS compartment are consistent with our previous characterisation of the PVS around the ventral aspect of the MCA, providing preliminary evidence that we are successfully capturing PVS fluid movement at a more downstream target within the glymphatic pathway.Discussion
In this pilot study, we have non-invasively assessed fluid movement in a downstream compartment of the PVS in the rat brain. We have preliminary evidence to suggest that arterial pulsation may drive fluid movement in the PVS and could be a major contributor to glymphatic inflow. This technique could serve as a potential early biomarker of perivascular impairment and therefore neurodegenerative diseases such as AD.Acknowledgements
This work is supported by the Medical Research Council (MR/K501268/1) and the EPSRC-funded UCL Centre for Doctoral Training in Medical Imaging (EP/L016478/1) together with the Wellcome Trust and Royal Society. DT is supported by the UCL Leonard Wolfson Experimental Neurology Centre (PR/ylr/18575).References
[1] Jessen, N., Munk, A., Lundgaard, I. and Nedergaard, M. (2015). The Glymphatic System: A Beginner’s Guide. Neurochemical Research, 40(12), pp.2583-2599.
[2] Benveniste, H., Liu, X., Koundal, S., Sanggaard, S., Lee, H. and Wardlaw, J. (2018). The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology, pp.1-14.
[3] Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11(26):1–32.
[4] Peng, W., Achariyar, T., Li, B., Liao, Y., Mestre, H., Hitomi, E., Regan, S., Kasper, T., Peng, S., Ding, F., Benveniste, H., Nedergaard, M. and Deane, R. (2016). Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiology of Disease, 93, pp.215-225.
[5] Harrison, I., Siow, B., Akilo, A., Evans, P., Ismail, O., Ohene, Y., Nahavandi, P., Thomas, D., Lythgoe, M. and Wells, J. (2018). Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. eLife, 7.
[6] Rodríguez-Contreras, A., Shi, L. and Fu, B. (2014). A Method to Make a Craniotomy on the Ventral Skull of Neonate Rodents. Journal of Visualized Experiments, (87).
Figures