3296
Measuring white matter injury in children with demyelinating syndromes as revealed by non-Gaussian diffusion imaging1Neuroscience and Mental Health, The Hospital for Sick Children, Toronto, ON, Canada, 2Department of Pediatrics, The Hospital for Sick Children, Toronto, ON, Canada
Synopsis
Characterizing whole brain white matter structure in children with demyelinating syndromes (e.g. multiple sclerosis (MS) and neuromyelitis optica spectrum disorders (NMOSD), and myelin oligodendrocyte glycoprotein antibody related disorders (MOG)) may shed light on patterns of injury which are not apparent using conventional imaging techniques. We evaluated group differences in non-Gaussian diffusion data between 26 healthy control, 17 MS and 17 MOG/NMOSD children. We show white-matter microstructure differences between healthy controls and MS patients in areas associated with oculomotor function. Specifically, we show lower axonal density and myelin volume along optic radiations in MS patients than controls or NMOSD/MOG patients.
Introduction
White matter microstructural changes occur in youth with MS and monophasic demyelinating disorders(1–3), affecting growth trajectory and cognitive outcomes even after one demyelinating event. Diffusion tensor imaging has been extensivly used in this population, however this method lacks mircrostructure and pathological specificity. Diffusion kurtosis imaging (DKI) is sensitive to microstructural changes associated with demyelination in an animal model (4) and brain maturation in healthy infants (5). Here we use DKI (6, 7) in combination with intra- and extra-cellular microstructure parameters from the White Matter Tract Integrity (WMTI) model (8) to measure white matter microstructure changes (such as axon density and myelin volume) in youth with demyelinating syndromes and healthy controls.Methods
Participants: Twenty-six healthy control children (15F;15.4 ± 2.15 years), 17 children with multiple sclerosis (MS) (11F; 16.9 ± 1.16 years; 13.7 ± 2.52 age of symptom onset) and 17 children with myelin oligodendrocyte glycoprotein antibody related disorders (MOG) and neuromyelitis optica spectrum disorders (NMOSD) (14F; 12.8 ± 2.91 years; 10.3 ± 3.18 age of symptom onset) were recruited.
Image acquisition: MRI images were acquired using a 3T Siemens Prisma system (Siemens Medical Solutions, Erlangen, Germany). Three sets diffusion-weighted images were acquired along 35, 45 and 66 directions for b-value of 1000,1600 and 2600 s/mm2 respectively with echo planar imaging (EPI) sequence with TR=3800 ms, TE=73.0 ms, FOV=244x244mm, 70 slices, slice thickness=2.0 mm, no gap, bandwidth=1952 Hx/pixel, 2xphase encoding polarities (Anterior->Posterior/Posterior->Anterior).
Analysis: Data was preprocessed using DESIGNER (9), which included denoising and Rician bias correction within MRtrix (Version 3.0 rc2) (10), Gibbs ringing correction (11), EPI distortion correction using topup (12), eddy current and motion correction using eddy in FSL (Version 5.0.11) (13) and outlier detection before iterative parameter estimation. White matter microstructure parameters were calculated using weighted linear least squares estimation (8, 14). These parameters included: fractional anisotropy (FA), mean diffusivity (MD), mean kurtosis (MK), intra- and extra-axonal diffusion tensors (IAS and EAS), axonal water fraction (AWF) and tortuosity (TORT) of the extra-axonal space.
Voxelwise analysis using tract-based spatial statistics (TBSS; (15)) was used to perform statistical analysis on the above parameters to test differences between our groups using age, age of diagnosis and gender as covariates using randomize (16) with 5000 permutations with threshold-free cluster enhancement and a statistical significance level set at p<0.05, corrected for multiple comparisons.
Results
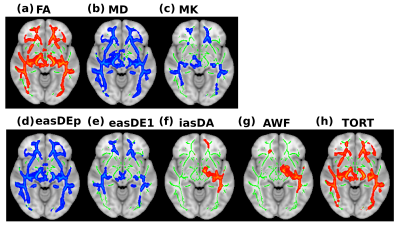
Figure 1 demonstrates the differences between healthy control children and MS patients in a number of DKI and WMTI metrics. There were no significant differences found between healthy control children and the MOG/NMOSD group across all variables tested. Voxelwise differences showed reduced (a) FA (b) AWF (c) axonal diffusivity (iasDa) and (d) TORT of extra-axonal space in MS patients. Voxelwise differences showed increased (a) MD (b) MK (c) EAS diffusivity in direction along the fibers (easDe1) (d) EAS diffusivity in perpendicular to the fibers (easDep) in MS patients. Areas where differences were found included areas known for oculomotor function, such as, left and right inferior fronto-occipital fasciculus, superior longitudinal fasciculus, optic radiations, anterior thalamic radiation and genu. Lower measures of AWF and tortuosity are indicators of decreases in axonal density and myelin volume and changes within diffusivities in the intra and extra-axonal space provides indicators of changes in myelin and axonal geometry, respectively.Conclusion
Our preliminary findings show significant differences in white matter microstructure between healthy controls and MS patients, but not MOG/NMOSD patients. We demonstrated that MS patients have lower axonal density (AWF) and myelin volume (TORT) along the optic radiation, which could be related to clinical outcomes (e.g. increased number of brain lesions). This adds to literature showing white matter changes in youth with MS and highlights contributions of both axonal and myelin injury to these changes. Larger studies are needed to confirm these findings. Future research will evaluate the relationship of these findings to clinically salient outcomes such as vision and cognitionAcknowledgements
The authors thank Stephanie Grover, Cynthia De Medeiros, Tara Berenbaum, Danusha Nandamalavan and Austin Noguera for their assistance with participant recruitment and Jovanka Skocic, Tammy Rayner and Ruth Weiss for image acquisition. We would also like to thank Benjamin Ades-Aron and Els Feiremans part of the DESINGER team at NYU for their invaluable help.References
1. Longoni G, Brown RA, Momayyezsiahkal P, et al.: White matter changes in paediatric multiple sclerosis and monophasic demyelinating disorders. Brain 2017; 140:1300–1315.
2. Amato MP, Goretti B, Ghezzi A, et al.: Cognitive and psychosocial features in childhood and juvenile MS: Two-year follow-up. Neurology 2010; 75:1134–1140.
3. Amato MP, Krupp LB, Charvet LE, Penner I, Till C: Pediatric multiple sclerosis: Cognition and mood. Neurology 2016; 87:S82–S87.
4. Guglielmetti C, Veraart J, Roelant E, et al.: Diffusion kurtosis imaging probes cortical alterations and white matter pathology following cuprizone induced demyelination and spontaneous remyelination. Neuroimage 2016; 125:363–377.
5. Paydar A, Fieremans E, Nwankwo JI, et al.: Diffusional kurtosis imaging of the developing brain. Am J Neuroradiol 2014; 35:808–814.
6. Jensen JH, Helpern J a, Ramani A, Lu H, Kaczynski K: Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005; 53:1432–1440.
7. Jensen JH, Helpern JA: MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 2010; 23:698–710.
8. Fieremans E, Jensen JH, Helpern J a: White matter characterization with diffusional kurtosis imaging. Neuroimage 2011; 58:177–188.
9. Ades-Aron B, Veraart J, Kochunov P, et al.: Evaluation of the accuracy and precision of the diffusion parameter EStImation with Gibbs and NoisE removal pipeline. Neuroimage 2018; 183(August):532–543.
10. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E: Denoising of diffusion MRI using random matrix theory. Neuroimage 2016; 142:394–406.
11. Veraart J, Fieremans E, Jelescu IO, Knoll F, Novikov DS: Gibbs ringing in diffusion MRI. Magn Reson Med 2016; 76:301–314.
12. Andersson JLR, Skare S, Ashburner J: How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 2003; 20:870–888.
13. Andersson JLR, Sotiropoulos SN: An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016; 125:1063–1078.
14. Tabesh A, Jensen JH, Ardekani BA, Helpern J a: Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med 2011; 65:823–836.
15. Smith SM, Jenkinson M, Johansen-Berg H, et al.: Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31:1487–1505.
16. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE: Permutation inference for the general linear model. Neuroimage 2014; 92:381–397.
Figures