3048
Concomitant reduction of glymphatic flow and CBF in mouse model of Alzheimer’s disease1The University of Queensland, Brisbane, Australia, 2Xi'an Jiaotong University, Shaanxi, China
Synopsis
An impaired glymphatic system has been implicated in the accumulation of toxins such as amyloid beta (Ab) in Alzheimer’s disease. As both glymphatic flow and cerebral blood flow are driven by arterial pressure, we hypothesized that vascular dysfunction represented by reduced cerebral blood flow might contribute to reduced glymphatic flow. Our preliminary results indicate that aged AD mice have both reduced tissue glymphatic flow and reduced cerebral blood flow. Our results suggests that impairment of the glymphatic system in AD may be partly due to impaired cerebrovascular function.
Introduction
The glymphatic system is a para-vascular pathway for waste clearance via cerebrospinal fluid in the brain. Its impairment has been suggested to lead to accumulation of toxins in diseases such as Alzheimer’s disease (AD) 1. On the other hand, reduced cerebral blood flow (CBF) has been observed in both aging and AD patients, demonstrating deficits in neural and vascular functions. However, the relationship between CBF and glymphatic flow is largely unexplored. As glymphatic flow is driven by arterial pulsation, we hypothesized that its reduction would correlate with the vascular clearance pathway represented by CBFMethods
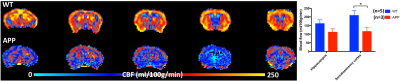
The study was approved by the animal ethic committee of the University of Queensland. Imaging of 12-14 month old AD (APPswe/PS1∆e9) mice and wild-type mice under 0.1mg/kg/h medetomidine and 0.25-0.5% isoflurane anaesthesia was conducted using a 9.4T MRI. CBF was measured by pseudo-continuous arterial spin labelling sequence (labelling time=3s, post-labeling delay=450ms, resolution=0.3x0.3x0.6mm3) and quantified by a kinetic model 2. The glymphatic flow was measured by dynamic 3D T1-weighted FLASH (TR/TE=21/2.66ms, 100-micron isotropic resolution) during the injection of Gd-DTPA into the cisterna magna. The Gd-enhanced time-course was normalized by the baseline scan, and the area-under-curve (AUC) from various brain regions was calculated.Results
A significant reduction (~44%) in CBF was found in the AD mice particularly in the cortex (Fig.1). Surprisingly, the Gd-contrast at the pituitary, an area showing early contrast enhancement (other than the injection site) and a first entry of Gd into the glymphatic system, was increased considerably in AD mice (Fig.2). On the contrary, the enhancement was comparable in other areas such as the cortex, thalamus and hippocampus. By normalizing the Gd-contrast enhanced AUC in gray matter areas by that of the pituitary, an overall reduction of Gd reaching the somatosensory cortex was calculated, being 3.82±0.29% (mean±SD, n=2) in the wild-type animals compared to 0.54±0.26% in AD mice (n=2), corresponding to a more than 5 fold reduction. Similarly, the Gd reaching the hippocampus reduced from 8.71±3.54% (wild-type) to 1.85±1.02% in AD mice.Discussion
This preliminary study demonstrates a reduction in both CBF and glymphatic flow in an AD mouse model. The large increase of Gd contrast in the pituitary recess in AD mice may be due to their enlarged ventricular space, which facilitates Gd inflow to the brain. By normalizing this “Gd input”, the proportion of avaliable Gd reaching brain tissue was found to be more limited in the AD animals. As the significant reduction in tissue glymphatic flow is coincident with reduced CBF in AD mice, the results suggest reduced vascular function may partly underlie the reduced glymphatic flow in AD. Further study is ongoing to determine the relationship of these measures, and to identify possible pathways driving the changes in AD.Acknowledgements
The project is supported by Mason Foundation.References
1 Plog BA, Nedergaard M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu Rev Pathol Mech Dis 2018; 13: annurev-pathol-051217-111018.
2 Hirschler L, Debacker CS, Voiron J, Köhler S, Warnking JM, Barbier EL. Interpulse phase corrections for unbalanced pseudo-continuous arterial spin labeling at high magnetic field. Magn Reson Med 2018; 79: 1314–1324.
Figures